Colitis in Small Animals
- Diseases of the Stomach and Intestines in Small Animals
- Canine Parvovirus
- Colitis in Small Animals
- Constipation and Obstipation in Small Animals
- Feline Enteric Coronavirus
- Gastric Dilation and Volvulus in Small Animals
- Gastritis in Small Animals
- Gastrointestinal Neoplasia in Small Animals
- Gastrointestinal Obstruction in Small Animals
- Gastrointestinal Ulcers in Small Animals
- Helicobacter Infection in Small Animals
- Hemorrhagic Gastroenteritis in Small Animals
- Inflammatory Bowel Disease in Small Animals
- Malabsorption Syndromes in Small Animals
The colon helps maintain fluid and electrolyte balance and absorb nutrients; it is also the major site of fecal storage until expulsion and provides an environment for microorganisms. Disruptions to normal colonic function lead to changes in both absorption and motility; clinically, this often manifests as large-bowel diarrhea. Approximately one-third of dogs with a history of chronic diarrhea have colitis. Chronic colitis is defined as inflammation of the colon that is present for ≥2 wk. Inflammation of the colon reduces the amount of water and electrolytes absorbed and changes colonic motility by suppressing the normal colonic contractions that mix and knead and by stimulating giant migrating contractions (ie, more powerful contractions that rapidly propel intestinal contents). Colitis has been classified into four forms: lymphocytic-plasmacytic, eosinophilic, neutrophilic, and granulomatous. Lymphocytic-plasmacytic is the most common form in both dogs and cats. Most dogs are middle-aged, and there is no sex predilection. There may be an association between colitis and perianal fistula, especially in German Shepherds. Cats with chronic colitis tend to be middle-aged and more commonly purebred. Typically, there is an increased number of lymphocytes and plasmocytes in the lamina propria (less frequently in the submucosa and muscularis).
Eosinophilic colitis is characterized by an increased number of eosinophils in the lamina propria. It is less common than lymphocytic-plasmacytic colitis, the animals tend to be younger, and it is more difficult to treat. Infectious agents, parasites, and food allergies may be inciting factors, but none has been proved. The CBC may reveal eosinophilia. Hypereosinophilic syndrome in cats is a variant of eosinophilic enteritis with eosinophilic involvement not only of the bowel but also of the liver, spleen, mesenteric lymph nodes, kidney, adrenal glands, and heart.
Granulomatous colitis (GC) is a rare, breed-specific inflammatory bowel disease of young Boxer dogs. It is seen as a segmental, thickened, partially obstructed segment of bowel (ileum and colon most commonly), characterized by the presence of macrophages and other inflammatory cells within the lamina propria. These macrophages are not periodic acid-schiff positive. Because of the histologic characteristics, it is important to exclude inflammation secondary to fungal disease, intestinal parasites, feline infectious peritonitis, and foreign material. Treatment remains controversial. Although surgery was previously recommended because GC was refractory to medical treatment and associated with high mortality rates, recent research using culture-independent molecular analysis has shown a correlation between GC and Escherichia coli invasion within colonic mucosal macrophages. Current treatment recommendations for GC require antimicrobials effective against E coli and that penetrate intracellularly, such as enrofloxacin.
Etiology and Pathophysiology:
Inflammation of the colon may be acute or chronic. In most cases, the inciting factors are unknown. Bacterial, parasitic, fungal, traumatic, uremic, and allergic causes have been postulated. Inflammation may be the result of a defect in mucosal immunoregulation. After initial mucosal injury, submucosal lymphocytes and macrophages become exposed to luminal antigens and subsequently trigger inflammation. An exaggerated reaction to dietary or bacterial factors within the lumen of the bowel, genetic predisposition, psychologic pathology affecting the neurologic or vascular supply to the colon, or sequelae of previous infectious or parasitic disease have also been implicated.
In acute colitis, there is mucosal infiltration with neutrophils and epithelial disruption and ulceration. Chronic colitis is most often characterized by mucosal infiltration of plasma cells and lymphocytes, fibrosis, and sometimes ulceration. Goblet cells are stimulated to secrete excessive quantities of mucus. Absorption of water and electrolytes is impaired, and motility is reduced. Inflammation disrupts intracellular tight junctions and reduces the transmucosal electrical potential difference, interrupting the ability of the colon to absorb sodium. Normal segmentation is inhibited; giant migrating muscular contractions proceed down the length of the colon and rapidly expel luminal contents. The inflamed bowel is more sensitive to stretch, and contents entering the colon stimulate strong migrating muscular contractions, an urge to defecate, and abdominal discomfort.
Fructooligosaccharides (FOSs) enhance colonic microflora and assist in the prevention and treatment of colonic disease. These complex carbohydrates are not digested in the small intestine. They are fermented by specific colonic bacteria that use them as an energy source. FOSs promote the growth of beneficial bacteria and inhibit growth of potentially harmful bacteria. They are responsible for the production of short-chain fatty acids (SCFAs).
SCFAs (acetate, propionate, butyrate) are an important energy source essential for maintenance of normal mucosal health. They help maintain intestinal motility and ameliorate intestinal inflammation. Alteration of fatty acids leads to mucosal atrophy and injury.
Clinical Findings:
The most common clinical sign of chronic colitis is large-bowel diarrhea, characterized by mucus, hematochezia, tenesmus, and occasionally pain when defecating. There is often an increased urgency and frequency of defecation, with decreased fecal volume per bowel movement. Weight loss and vomiting can occur but are uncommon; they are seen more often when small intestine is involved. Clinical signs may wax and wane. Initially, the clinical signs may be sporadic, but progression usually occurs. Physical examination is unremarkable in most cases. A thorough rectal examination may reveal rectal polyps or malignant neoplasms that can mimic signs of chronic colitis.
Diagnosis:
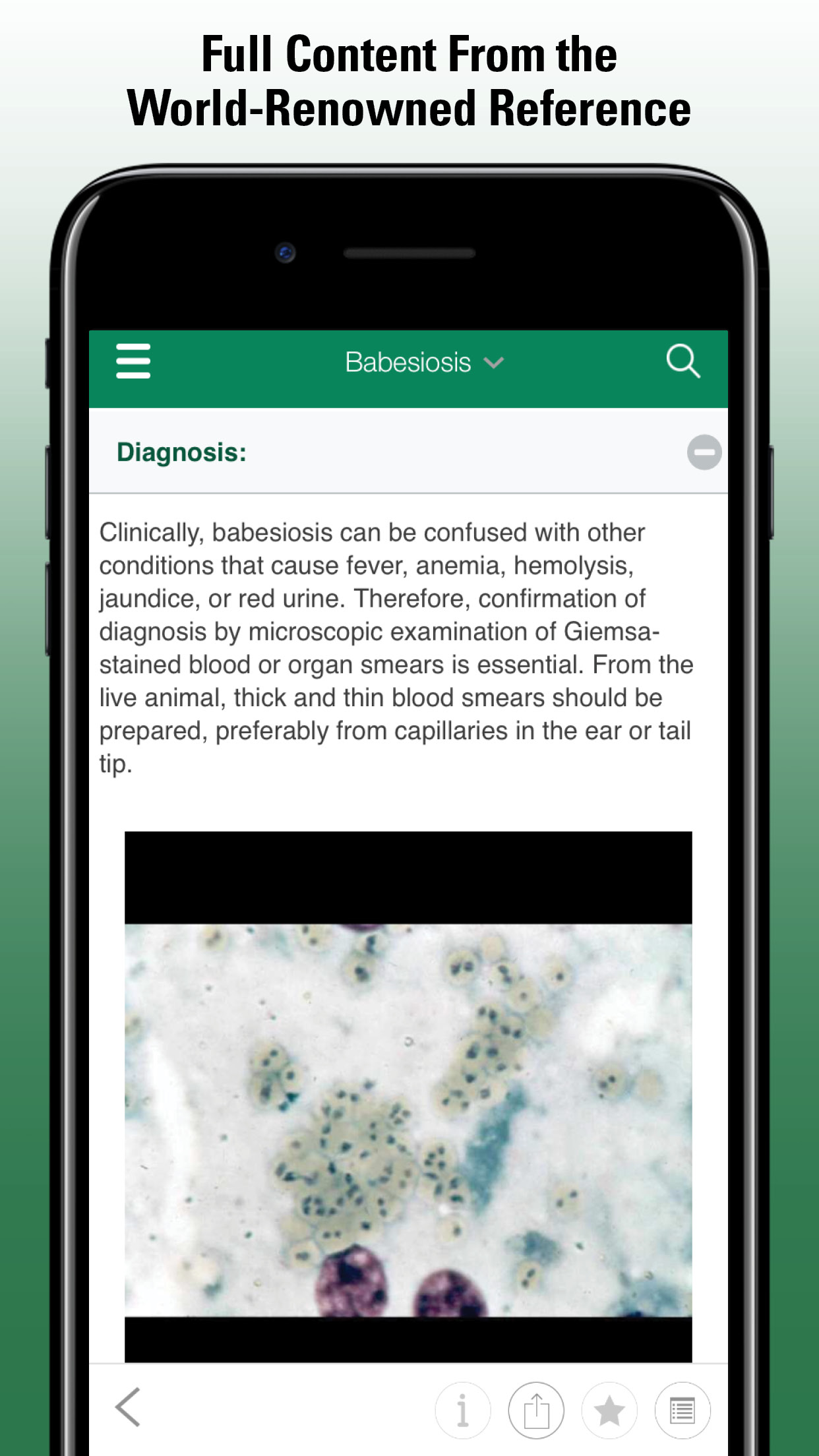
The initial approach should include a complete history and physical examination, including rectal palpation and evaluation of feces. Fecal smears for Giardia and fungal elements (Histoplasma capsulatum, Pythium insidiosum), fecal flotation for parasite identification (Trichuris vulpis in dogs, Tritrichomonas foetus in cats), and culture for bacteria (Campylobacter, Salmonella, Clostridium) are suggested in cases of chronic colitis. Rectal cytology is an important tool to exclude other causes of large-bowel diarrhea. It can reveal inflammatory cells, neoplastic cells, and certain infectious agents (eg, H capsulatum). Cases of suspected clostridial colitis (>5 endospores per field) should be confirmed by identifying Clostridium perfringens enterotoxin A and B in feces using a commercially available ELISA after a fecal bacterial culture is performed.
A dietary trial is recommended before pursuing more advanced diagnostics. If clinical signs persist, a CBC, biochemical profile, and urinalysis should be performed to exclude other diseases; however, in most cases of chronic colitis, the results are normal. For cats, feline leukemia virus/feline immunodeficiency virus testing is also recommended as well as a thyroid level if age appropriate. Routine abdominal radiographs are also usually normal. Contrast radiographs may occasionally demonstrate intraluminal narrowing, which could indicate an infiltrative disease process. Ultrasonography allows the visualization of colonic mucosa, localized lesions, and the size and echogenicity of lymph nodes.
Colonoscopy is indicated to visually inspect the mucosal surface of the colon and to obtain biopsy specimens. Preparation of the colon is essential to avoid missing small or subtle lesions because of residual fecal material on the mucosal surface. Food should be withheld for 24–48 hr before the procedure, followed by a combination of enemas and an oral colonic lavage solution. Several agents can be used to clean the bowel, such as sodium picosulfate and bisacodyl. Multiple samples from the cecum and ascending, transverse, and descending colon should be obtained, regardless of gross morphologic appearance. Because of poor correlation between gross appearance and histopathologic results, results should be interpreted in light of the physical examination and history. A normal mucosal biopsy or one with evidence of a hyperplastic mucosa, in conjunction with clinical signs supportive of large-intestinal diarrhea, is compatible with irritable bowel syndrome. Peripheral eosinophilia is invariably present in cats with hypereosinophilic syndrome.
Treatment and Control:
If possible, the inciting cause should be identified and eliminated. Food should be withheld for an initial 24–48 hr in animals with acute colitis in an effort to “rest” the bowel.
Because shedding of ova by whipworms is intermittent, therapeutic deworming (eg, fenbendazole 50 mg/kg/day, for 3 days, repeated in 3 wk and again in 3 mo if there is a positive response) should be done even if results of fecal examinations are negative.
Supplementing the diet with fiber (1–6 tsp of psyllium hydrophilic mucilloid or 1–4 tbsp of coarse wheat bran/feeding) improves diarrhea in many animals. Dietary fiber reduces free fecal water, prolongs luminal transit time (increasing the opportunity to absorb water), absorbs toxins, increases fecal bulk and stretches the colonic smooth muscle, and improves contractility. However, the addition of fiber alone rarely results in complete resolution of clinical signs of large-intestinal diarrhea in dogs, and beneficial effects may take as long as 6 wk to become evident. Over time, the fiber dose can be reduced or eliminated in some dogs and a standard dog food substituted without causing a return of the diarrhea.
Novel protein diets have effectively controlled clinical signs of colitis in both dogs and cats. The protein source used should be one to which the animal has not previously been exposed. In one study, clinical signs associated with lymphocytic-plasmacytic colitis resolved in all dogs within ~2 wk after feeding a low-residue, digestible, hypoallergenic diet (1 part low-fat cottage cheese and 2 parts boiled white rice). Thereafter, most dogs were maintained without recurrence of clinical signs on commercially available prescription diets they had not been previously fed. Currently, a number of commercially available diets contain rice with mutton or lamb, venison, or rabbit.
Hydrolyzed diets have also been effective in treatment of colitis. These specialized diets disrupt the protein structure sufficiently to remove any allergens and allergenic epitopes and, therefore, prevent immune recognition.
If feeding a high-fiber or novel protein diet is not beneficial, a commercial, low-residue diet may be tried, especially one that contains FOSs.
Cats with lymphocytic-plasmacytic colitis may respond to dietary management alone (eg, lamb and rice, horsemeat, or a commercially available diet). In one study, cats were initially treated with dietary fiber or with dietary fiber and pharmacologic intervention (prednisone, tylosin, or sulfasalazine). Most cats were eventually maintained on high-fiber diets or a highly digestible diet.
Metronidazole is considered one of the primary pharmacologic agents in chronic colitis in cats. Its therapeutic effects include antiprotozoal and antimicrobial activity and inhibition of some aspects of cell-mediated immunity. It is not usually used as a sole agent but rather in combination with either dietary management or another drug. Although metronidazole is well tolerated in both dogs and cats, adverse effects can occur (mostly neurologic, eg, nystagmus, ataxia, vestibular signs, seizures), either with chronic therapy or at high dosages. However, neurotoxicoses should be reversible within 5–7 days after treatment is discontinued.
Tylosin, a macrolide antibiotic used primarily in food animals, is useful in chronic enteropathies, because it interferes with bacterial adhesion to the mucosa and has some antibacterial and immunomodulating effects. It targets mainly facultative and obligate anaerobic gram-positive bacteria and some gram-negative bacteria. However, E coli and Salmonella are resistant to tylosin. Tylosin is well tolerated in both dogs and cats with minimal effects.
Clinical signs resolve more rapidly when anti-inflammatory medication is given, along with the change in diet. Sulfasalazine, prednisone or prednisolone, and azathioprine are used most commonly. Sulfasalazine is often used to treat lymphocytic-plasmacytic colitis in dogs (12.5 mg/kg, qid for 14 days, then 12.5 mg/kg, bid for 28 days). Longterm use is discouraged, because it predisposes to keratoconjunctivitis sicca. Sulfasalazine is a prostaglandin synthetase inhibitor and has antileukotriene activity. It consists of mesalamine linked to sulfapyridine in an azochemical bond; this linkage prevents absorption in the upper GI tract and allows most of the drugs to be transported to the large intestine. Once it has reached the large intestine, it is metabolized by cecal and colonic bacteria, releasing both components. Mesalamine acts locally to reduce colonic mucosal inflammation. Sulfapyridine is believed to be systemically absorbed and therefore does not have any local therapeutic effect in colitis but is blamed for the adverse effects of sulfasalazine. Salicylates are metabolized in the liver by hepatic enzymatic processes involving glucuronyl transferase. Because cats are deficient in this enzymatic pathway, salicylates have prolonged half-lives in this species. Therefore, sulfasalazine is not used as the drug of choice in colitis in cats because of the risk of salicylate toxicity.
Glucocorticoids, in combination with dietary management and metronidazole, are the treatment of choice for chronic colitis in cats. They may be introduced into the therapeutic plan for dogs when the previously discussed therapies are not successful or if the 5-aminosalicylates result in adverse effects. If used in combination with sulfasalazine or metronidazole, prednisone may be given at a reduced dosage. Prednisone should be started at 2 mg/kg/day, PO; for 2 wk after clinical signs resolve, the dosage should be reduced by 25% every 2–4 wk, which can usually maintain remission.
Cats usually tolerate glucocorticoids very well; adverse effects are common in dogs and include polyuria, polydipsia, polyphagia, GI bleeding, increased susceptibility to infection, iatrogenic hyperadrenocorticism, and pituitary-adrenocortical suppression.
Budesonide is a nonhalogenated glucocorticoid used in treatment of Crohn’s disease in people. Budesonide undergoes significant first-pass metabolism in the liver; theoretically, this should reduce the adverse effects often seen with traditional glucocorticoids, because little of the active drug is systemically available. In one study of 10 healthy dogs, the pituitary-adrenocortical axis was suppressed, but no other adverse effects were seen.
Immunosuppressive drugs are mostly used in combination with glucocorticoids when the response is not satisfactory with the latter alone. The most commonly used are azathioprine and chlorambucil in dogs and cats. Azathioprine (2 mg/kg/day, and then tapered), alone or in combination with prednisone, has been used to control clinical signs associated with lymphocytic-plasmacytic colitis. Azathioprine may be considered in cases that are poorly responsive to prednisone or to prednisone with sulfasalazine. The serious adverse effects of azathioprine in cats (myelosuppression and hepatotoxicity) limit its use in feline colitis. Instead, chlorambucil (0.1–0.2 mg/kg or 1 mg/cat, daily initially until clinical signs are markedly improved, which may require 4–8 wk) is used in cats in combination with prednisone if needed.
Cyclosporine has been effective in steroid-refractory cases of colitis, but it has not been evaluated in cats. Adverse effects include GI disturbances, gingival disease, and alopecia.
Some animals also require short-term use of motility modifiers until inflammation is controlled. Loperamide (0.1–0.2 mg/kg, bid-qid) stimulates segmental activity and slows passage of fecal contents. It also decreases colonic secretion, enhances salt and water absorption, and increases anal sphincter tone. It is contraindicated in cases of infectious colitis (eg, caused by Salmonella, Campylobacter, or Clostridium).
Prognosis:
The short-term prognosis for chronic colitis is good for both dogs and cats. However, longterm prognosis for complete resolution without relapses appears poor. Most cases of inflammatory bowel disease are not curable, and some form of treatment will likely be necessary longterm. For some animals, especially cats, longterm management of chronic colitis may be possible with diet alone.
Most cases of idiopathic lymphocytic-plasmacytic colitis respond to appropriate dietary and medical changes. Stricture formation and extensive fibrosis warrant a more guarded prognosis. Eosinophilic colitis in dogs responds favorably to controlled diets and glucocorticoid therapy. In cats, the prognosis is more guarded, and more aggressive treatment with immunosuppressive agents is required. Hypereosinophilic syndrome is a progressive, fatal disease that has no effective treatment in animals.
Histiocytic colitis of Boxers carries a grave prognosis unless treatment is started early in the course of the disease. The immunoproliferative enteropathy of Basenjis also carries a poor prognosis; most dogs die within 2 yr of diagnosis, although some have been reported to live as long as 5 yr. Similarly, the prognosis for the diarrheal syndrome reported in Lundehunds is also poor.
- Diseases of the Stomach and Intestines in Small Animals
- Canine Parvovirus
- Colitis in Small Animals
- Constipation and Obstipation in Small Animals
- Feline Enteric Coronavirus
- Gastric Dilation and Volvulus in Small Animals
- Gastritis in Small Animals
- Gastrointestinal Neoplasia in Small Animals
- Gastrointestinal Obstruction in Small Animals
- Gastrointestinal Ulcers in Small Animals
- Helicobacter Infection in Small Animals
- Hemorrhagic Gastroenteritis in Small Animals
- Inflammatory Bowel Disease in Small Animals
- Malabsorption Syndromes in Small Animals