Plasmodium Infection in Poultry
- Bloodborne Organisms
- Overview of Bloodborne Organisms in Poultry
- Aegyptianellosis in Poultry
- Isosporiasis in Poultry
- Filariasis in Poultry
- Haemoproteus Infection in Poultry
- Leucocytozoonosis in Poultry
- Plasmodium Infection in Poultry
- Other Bloodborne Organisms in Poultry
Plasmodium spp infect a wide variety of domestic and wild birds in most areas of the world. Infection is often not species specific. Thirty-five species of Plasmodium are considered valid species. P gallinaceum, P juxtanucleare, and P durae are the most pathogenic species found in poultry. P gallinaceum infects chickens in Asia and Africa and causes low mortality in indigenous chickens; however, rates may be as high as 80%–90% in commercial birds. P juxtanucleare infects chickens and turkeys in Asia, Africa, and South America; most infections are mild or asymptomatic. P durae infects turkeys and gallinaceous birds other than chickens in Africa; mortality in turkeys can approach 100%. P hermani infects turkeys and bobwhite quail. Clinical malaria has not been reported from poultry in North America, but indigenous wild turkeys can become infected with at least four different Plasmodium species. The most common species affecting wild birds is P relictum, which has been found in at least 360 species of birds. Asymptomatic infections in endemic or introduced birds can be spread via mosquitoes and cause fatal disease in introduced (eg, zoo birds) or resident (eg, Hawaiian avifauna) birds, respectively. Passerine birds commonly carry the organism asymptomatically. Cold-climate species held ouside their natural range are particularly susceptible to develop clinical disease (eg, penguins, snowy owls, and gyrfalcons in captivity). Invertebrate hosts are ornithophilic mosquitoes, usually Culex, Culiseta, or Aedes spp.
Clinical Findings, Lesions, and Diagnosis:
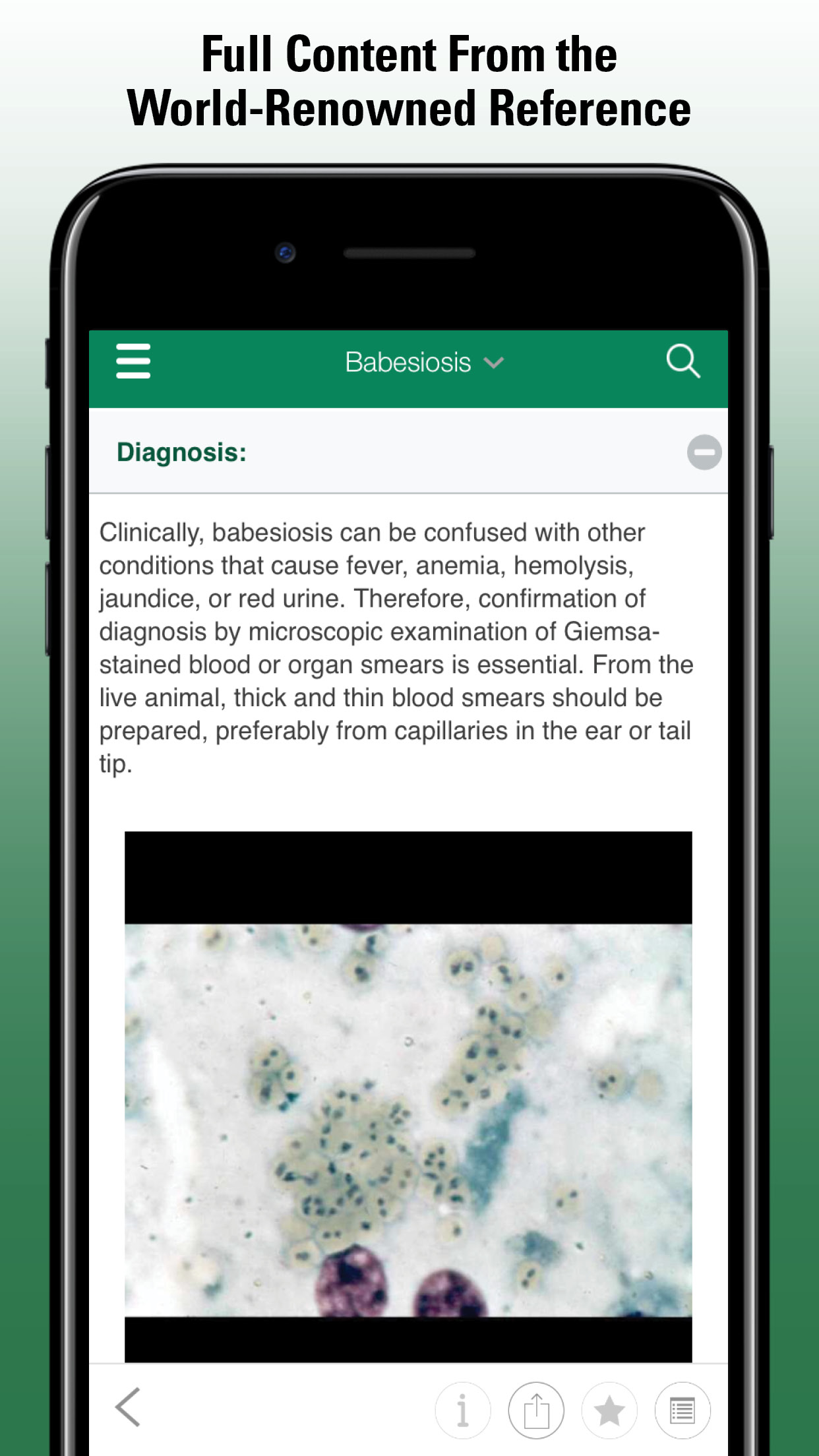
Infection with Plasmodium spp may be nonclinical or cause illness characterized by weakness, lassitude, dyspnea, anemia, abdominal distention, increased right heart weight, ocular hemorrhage, biliverdinuria, and death. Death results from severe anemia or blockage of capillaries in the brain, lung, or other vital organs by exoerythrocytic meronts in endothelial cells. The liver and spleen are markedly enlarged and often discolored (dark brown to black). Pigmented parasites including meronts are found in both immature and mature RBCs. Infrequently, parasites are found in thrombocytes and WBCs. In birds that die acutely, organisms may be sparse or absent in blood, but numerous meronts can be found in capillaries by histology or examination of squash or impression smears of brain, lung, liver, and spleen. Serologic and molecular diagnostic methods exist but are not available commercially. Serology and PCR can detect infection when parasites are too few to be identified in blood smears.
Treatment and Control:
Therapy is variably effective in treating infected birds or flocks. Persistent parasitemia or relapse may occur during and after treatment. Birds that survive initial infections may be refractory to subsequent infections. Prevention of exposure to mosquitoes by appropriate housing is essential.
No antimalarial drug is commercially available or approved to treat poultry flocks. However, a mixture of trimethoprim and sulfaquinoxaline in the feed for a 5-day period has been shown to be efficacious against experimentally induced P gallinaceum malaria in chickens. An experimental study on the pathogenicity and chemotherapy of P durae suggested that a combination of sulfamonomethoxine and sulfachloropyrazine could be an effective therapy; halofuginone was suggested for chemoprophylaxis in endemic areas. Chloroquine administered by gavage at 50 mg/kg in Leghorn chickens experimentally infected with P juxtanucleare may have reduced parasitemia.
In caged birds and penguins, chloroquine (10 mg/kg) and primaquine (0.3–1 mg/kg) is given orally and followed by administration of chloroquine (5 mg/kg) 6, 24, and 48 hr later. Chloroquine in drinking water (250 mg/120 mL) has also been used in songbirds. Grape or orange juice can disguise chloroquine’s bitterness. Treatment including both primaquine and chloroquine is recommended over chloroquine alone, because only primaquine is active against the tissue schizonts. Chloroquine has activity against erythrocytic schizonts and gametocytes. Primaquine also has activity against erythrocytic gametocytes. When aliquoting the medications, note that a 500-mg tablet of chloroquine contains 300 mg of active base, and a 26-mg primaquine tablet contains 15 mg of active base.
In raptors, control of the disease has been achieved by oral administration of mefloquine (30 mg/kg) repeated 12, 24, and 48 hr after the initial dose. Alternatively, a combination of chloroquine (25 mg/kg) and primaquine (1.3 mg/kg) can be given orally and is followed by the administration of chloroquine (15 mg/kg) 12, 24, and 48 hr later. In endemic areas, mefloquine once a week (30 mg/kg) has been used successfully for chemoprophylaxis in large falcons.
In a DNA vaccine trial in captive African black-footed penguins, parasitemia and clinical disease were successfully reduced.
Resources In This Article
- Bloodborne Organisms
- Overview of Bloodborne Organisms in Poultry
- Aegyptianellosis in Poultry
- Isosporiasis in Poultry
- Filariasis in Poultry
- Haemoproteus Infection in Poultry
- Leucocytozoonosis in Poultry
- Plasmodium Infection in Poultry
- Other Bloodborne Organisms in Poultry