Babesiosis
Babesiosis is caused by intraerythrocytic protozoan parasites of the genus Babesia. Transmitted by ticks, babesiosis affects a wide range of domestic and wild animals and occasionally people. Although the major economic impact of babesiosis is on the cattle industry, infections in other domestic animals, including horses, sheep, goats, pigs, and dogs, assume varying degrees of importance throughout the world.
Two important species in cattle—B bigemina and B bovis—are widespread in tropical and subtropical areas and are the focus of this discussion. However, because there are many common features of the diseases caused by different Babesia, much of this information can be applied to other species.
Transmission and Epidemiology:
The main vectors of B bigemina and B bovis are 1-host Rhipicephalus (Boophilus) spp ticks, in which transmission occurs transovarially. Although the parasites can be readily transmitted experimentally by blood inoculation, mechanical transmission by insects or during surgical procedures has no practical significance. Intrauterine infection has also been reported but is rare.
In Rhipicephalus spp ticks, the blood stages of the parasite are ingested during engorgement and undergo sexual and asexual multiplication in the replete female, infecting eggs and subsequent parasitic stages. Transmission to the host occurs when larvae (in the case of B bovis) or nymphs and adults (in the case of B bigemina) feed. The percentage of larvae infected can vary from 0–50% or higher, depending mainly on the level of parasitemia of the host at the time the female ticks engorge. Under field conditions, the rate of tick transmission is generally higher for B bigemina than for B bovis.
In endemic areas, three features are important in determining the risk of clinical disease: 1) calves have a degree of immunity (related both to colostral-derived antibodies and to age-specific factors) that persists for ~6 mo, 2) animals that recover from Babesia infections are generally immune for their commercial life (4 yr), and 3) the susceptibility of cattle breeds to ticks and Babesia infections varies; eg, Bos indicus cattle tend to be more resistant to ticks and the effects of B bovis and B bigemina infection than Bos taurus–derived breeds. At high levels of tick transmission, virtually all calves become infected with Babesia by 6 mo of age, show few if any clinical signs, and subsequently become immune. This situation can be upset by either a natural (eg, climatic) or artificial (eg, acaricide treatment or changing breed composition of herd) reduction in tick numbers to levels such that tick transmission of Babesia to calves is insufficient to ensure all are infected during this critical early period. Other circumstances that can lead to clinical outbreaks include the introduction of susceptible cattle to endemic areas and the incursion of Babesia-infected ticks into previously tick-free areas. Strain variation in immunity has been demonstrated but is probably not of practical significance in the field.
Clinical Findings and Pathogenesis:
B bovis is a much more virulent organism than B bigemina. With most strains of B bigemina, the pathogenic effects relate more directly to erythrocyte destruction. With virulent strains of B bovis, a hypotensive shock syndrome, combined with generalized nonspecific inflammation, coagulation disturbances, and erythrocytic stasis in capillaries, contribute to the pathogenesis.
The acute disease generally runs a course of ~1 wk. The first sign is fever (frequently ≥106°F [41°C]), which persists throughout, and is accompanied later by inappetence, increased respiratory rate, muscle tremors, anemia, jaundice, and weight loss; hemoglobinemia and hemoglobinuria occur in the final stages. CNS involvement due to adhesion of parasitized erythrocytes in brain capillaries can occur with B bovis infections. Either constipation or diarrhea may be present. Late-term pregnant cows may abort, and temporary infertility due to transient fever may be seen in bulls.
Animals that recover from the acute disease remain infected for a number of years with B bovis and for a few months in the case of B bigemina. No clinical signs are apparent during this carrier state.
Lesions:
Lesions (particularly with B bovis) include an enlarged and friable spleen; a swollen liver with an enlarged gallbladder containing thick granular bile; congested, dark-colored kidneys; and generalized anemia and jaundice. Most clinical cases of B bigemina have hemoglobinuria, but this is not invariably the case with B bovis. Other organs, including the brain and heart, may show congestion or petechiae.
Diagnosis:
Clinically, babesiosis can be confused with other conditions that cause fever, anemia, hemolysis, jaundice, or red urine. Therefore, confirmation of diagnosis by microscopic examination of Giemsa-stained blood or organ smears is essential. From the live animal, thick and thin blood smears should be prepared, preferably from capillaries in the ear or tail tip.
Smears of heart muscle, kidney, liver, lung, brain, and from a blood vessel in an extremity (eg, lower leg) should be taken at necropsy.
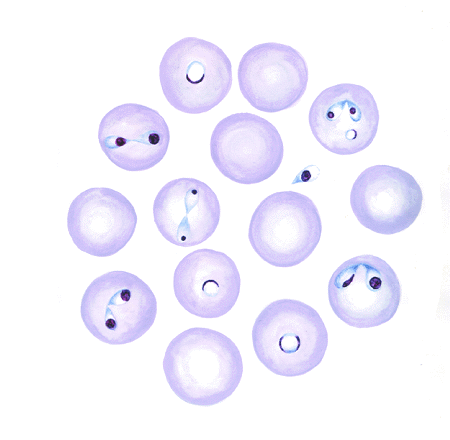
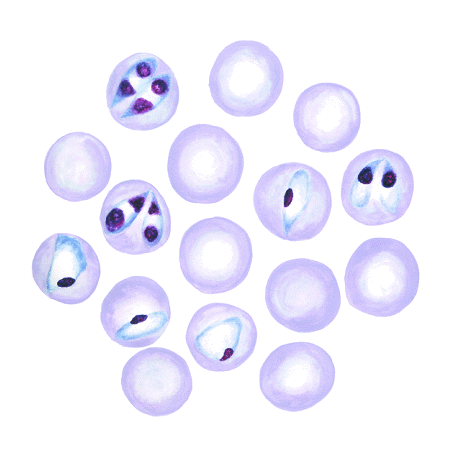
Microscopically, the species of Babesia involved can be determined morphologically, but expertise is required, especially in B bovis infections in which few organisms are present. B bovis is small, with the parasites in paired form at an obtuse angle to each other and measuring ~1–1.5 × 0.5–1 µm. B bigemina is larger (3–3.5 × 1–1.5 µm), with paired parasites at an acute angle to each other. Single forms of both parasites are also commonly seen.
A number of serologic tests have been described for detection of antibodies to Babesia in carrier animals. The most commonly used are the indirect fluorescent antibody test and ELISA. A commercially produced ELISA for B bigemina is available. PCR and real-time PCR assays capable of detecting extremely low parasitemias, as occur in carrier animals, and differentiating isolates have also been described. A procedure that may occasionally be justified to confirm infection in suspected carrier animals is the subinoculation of blood (~500 mL) into a fully susceptible animal, preferably a splenectomized calf, and subsequent monitoring of the recipient for infection.
Treatment and Control:
A variety of drugs have been used to treat babesiosis in the past, but only diminazene aceturate and imidocarb dipropionate are still in common use. These drugs are not available in all endemic countries, or their use may be restricted. Manufacturers' recommendations for use should be followed. For treating cattle, diminazene is given IM at 3.5 mg/kg. For treatment, imidocarb is given SC at 1.2 mg/kg. At a dosage of 3 mg/kg, imidocarb provides protection from babesiosis for ~4 wk and will also eliminate B bovis and B bigemina from carrier animals.
Supportive treatment is advisable, particularly in valuable animals, and may include the use of anti-inflammatory drugs, corticosteroids, and fluid therapy. Blood transfusions may be life-saving in very anemic animals.
Vaccination using live, attenuated strains of the parasites has been used successfully in a number of countries, including Argentina, Australia, Brazil, Israel, South Africa, and Uruguay. The vaccine is provided in either a chilled or frozen form. One vaccination produces adequate immunity for the commercial life of the animal; however, vaccine breakdowns have been reported. Several recombinant antigens have been shown experimentally to induce some immunity, but commercial vaccines are not yet available.
Although controlling or complete eradication of the tick vector can break the transmission cycle, this approach is rarely feasible in the longterm and can lead to large, susceptible populations in endemic areas with consequent risk of outbreaks of disease in naive animals.
Zoonotic Risk:
A number of cases of human babesiosis have been reported. The rodent parasite B microti and the cattle parasite B divergens are the most commonly implicated species in North America and Europe, respectively. However, B duncani, B venatorum, B conradae, and some less well-defined species have also been incriminated. The reservoir hosts and vectors of some of these species are not necessarily known with any certainty. Human Babesia infections are acquired via bites from infected ticks or through contaminated blood from an infected transfusion donor. Cases reported in splenectomized or otherwise immunocompromised individuals are often fatal.
Other Important Babesia of Domestic Animals
More than 100 species of Babesia have been isolated from domestic animals and wildlife. The following are indicative of those affecting domestic animals, but the list is far from complete.
Cattle:
B divergens and B major are two temperate-zone species with features comparable to those of B bovis and B bigemina, respectively. B divergens is a small, pathogenic Babesia of considerable importance in the British Isles and northwest Europe, whereas B major is a large Babesia of lower pathogenicity. B divergens is transmitted by Ixodes ricinus, and B major by Haemaphysalis punctata.
Horses:
Equine babesiosis is caused by Theileria (formerly Babesia) equi or B caballi. T equi is a small parasite and is more pathogenic than B caballi. T equi was reclassified as a Theileria (see Theileriases) in 1998. Equine babesiosis is found in Africa, Europe, Asia, South and Central America, and the southern USA. It is transmitted by ticks of the genera Rhipicephalus, Dermacentor, and Hyalomma. Intrauterine infection, particularly with T equi, is also relatively common.
Sheep and Goats:
Although small ruminants can be infected by several species of Babesia, the two most important species are B ovis and B motasi, transmitted by Rhipicephalus bursa and Haemaphysalis spp, respectively. Infection is of importance in the Middle East, southern Europe, and some African and Asian countries.
Pigs:
B trautmanni has been recorded as causing severe disease in pigs. This parasite has been reported from Europe and Africa. Another species, B perroncitoi, is of similar pathogenicity but apparently has a limited distribution in the areas mentioned above. The vectors of these Babesia have not been clarified, although Rhipicephalus spp have been shown to transmit B trautmanni.
Dogs and Cats:
Babesia species have been reported in dogs from most regions. These include B canis, B vogeli, and B rossi. B canis is transmitted by Dermacentor reticularis in Europe, B vogeli by Rhipicephalus sanguineus in tropical and subtropical countries, and B rossi by Haemaphysalis leachi in South Africa. Consequences of Babesia infection vary from a mild, transient illness to acute disease that rapidly results in death.
B gibsoni is the other important Babesia of dogs and is a much smaller parasite. It has a more limited distribution and characteristically causes a chronic disease with progressive, severe anemia that is not readily treated with normal babesiacides.
Illness of varying severity due to B felis in domestic cats has mostly been reported in southern Africa. Sporadic cases associated with other Babesia species have been reported elsewhere. An unusual feature of B felis is its lack of response to the normal babesiacides. However, primaquine phosphate (0.5 mg/kg, IM, twice with a 24-hr interval) is reported to be effective.