Overview of Colic in Horses
- Colic in Horses
- Overview of Colic in Horses
- Diseases Associated with Colic by Anatomic Location
In its strictest definition, the term “colic” means abdominal pain. Throughout the years, it has become a broad term for a variety of conditions that cause a horse to exhibit clinical signs of abdominal pain. Consequently, it is used to refer to conditions of widely varying etiologies and severity. To understand these etiologies, make a diagnosis, and initiate appropriate treatments, veterinarians must first appreciate the clinically relevant aspects of equine GI anatomy, the physiologic processes involved in movement of ingesta and fluid along the GI tract, and the extreme sensitivity of the horse to the deleterious effects of the structural components of the bacteria that reside within the lumen of the intestine.
GI Anatomy
The horse is a monogastric animal, with a relatively small stomach (capacity 8–10 L) that is located on the left side of the abdomen beneath the rib cage. The junction of the distal esophagus and the cardia is a functional 1-way valve, permitting gas and fluid to move into the stomach but not out. Consequently, conditions that impede the normal aboral movement of gas and fluid through the small intestine may result in severe dilation and rupture of the stomach. Because of its position, the stomach is difficult to visualize with radiography or ultrasonography in large adult horses. The smaller size of the foal, however, permits assessment of gastric emptying by contrast radiography.
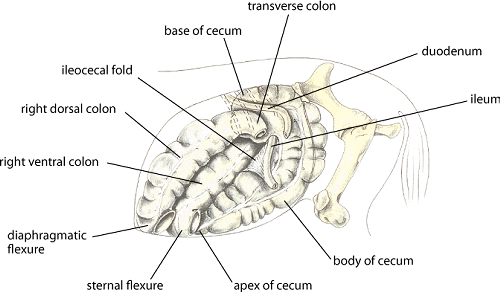
The small intestine comprises the duodenum, jejunum, and ileum, with the latter joining the cecum at a distinct ileocecal junction. The duodenum is positioned primarily dorsally on the horse’s right side, where it is suspended from the dorsal body wall by a short mesentery of 3–5 cm. Consequently, the duodenum is not involved in small-intestinal displacements involving the mesentery (volvulus). At the base of the cecum in the right paralumbar fossa region, the duodenum turns toward the midline. It is at this point that the duodenum, if distended with gas or fluid (eg, in horses with proximal enteritis), can be felt on rectal examination.
As the small intestine reaches the dorsal midline, it turns anteriorly, its mesentery lengthens, and it becomes known as the jejunum. The characteristic long mesentery allows loops of the jejunum to rest on the contents of the ventral portion of the abdomen. The jejunum is ~65 ft (19.5 m) long; its length, coupled with its long mesentery, allow it to be involved in small-intestinal volvulus and incarcerations. At the end of the jejunum, the wall of the intestine becomes more muscular, the lumen is narrowed, and an additional mesenteric attachment becomes apparent. The last 18 in. (45 cm) of the small intestine, the ileum, joins the cecum at its dorsal medial aspect. This junction is identified by the attachment of the ileocecal fold from the ileum to the dorsal band of the cecum. This ileocecal fold is used as a landmark to locate the ileum during abdominal surgery.
From the ileum, the ingesta enters the cecum, a large, blind-ended fermentation vat situated primarily on the horse’s right side, extending from the region of the paralumbar fossa to the xiphoid cartilage on ventral midline. The cecum is 4–5 ft (1.2–1.5 m) long and can hold 27–30 L of feed and fluid. Under the influence of the cecal musculature, the ingesta in the cecum is massaged, mixed with microorganisms capable of digesting cellulose, and eventually passed through the cecocolic opening into the right ventral colon. The attachment of the cecum to the dorsal body wall is wide, thus minimizing the likelihood the cecum can become displaced or twisted on its own.
The right ventral colon is divided into sacculations that help mix and retain plant fibers until they are digested. It is positioned on the ventral aspect of the abdomen, extending from the flank region to the rib cage. The ventral colon then turns toward the left, becoming the sternal flexure and then the left ventral colon. The left ventral colon, which also is large and sacculated, passes caudally to the left flank area. Near the pelvic region, the diameter of the colon decreases markedly, and the colon folds back on itself. This region, called the pelvic flexure, is the initial portion of the unsacculated left dorsal colon. Presumably because of the abrupt decrease in diameter, the junction between the left ventral colon and pelvic flexure is the most common location for impactions.
The diameter of the dorsal colon is largest either at its diaphragmatic flexure or in the right dorsal colon. There are no sacculations in either the left or right portion of the dorsal colon. The right dorsal colon is closely attached to the right ventral colon by a short intercolic fold and to the body wall by a tough, common mesenteric attachment with the base of the cecum. In contrast, neither the left ventral nor left dorsal colons are attached directly to the body wall, allowing these portions of the colon to become displaced or twisted.
Ingesta moves from the large right dorsal colon into the short transverse colon, which has a diameter of ~10 cm and is fixed firmly to the most dorsal aspect of the abdominal cavity by a strong, short, fibrous mesentery. The transverse colon is located cranial to the cranial mesenteric artery. Finally, the ingesta enters the sacculated descending colon, which is 10–12 ft (3–3.6 m) long.
Blood Supply to the GI Tract
The celiac and cranial mesenteric arteries (branches of the abdominal aorta) supply blood to the GI tract. The celiac artery supplies arterial blood to the stomach, pancreas, liver, spleen, and the first portion of the duodenum. The cranial mesenteric artery supplies arterial blood to the remaining portion of the duodenum; to all of the jejunum, ileum, cecum, large colon, and transverse colon; and to the first portion of the descending colon. Because the large colon is attached to the body wall only in the region near the cranial mesenteric artery, the blood supplying all portions of the colon must traverse the entire length of the colon. The pelvic flexure receives its blood supply from two branches of the cranial mesenteric artery; one branch supplies the right and left dorsal colons before reaching the pelvic flexure, and the other branch supplies the right and left ventral colons before reaching the pelvic flexure. Thus, volvulus of the large colon near the junction of the colon and cecum may impede the flow of blood to the entire left colon.
The major branches of the cranial mesenteric artery can be damaged by the migrating forms of Strongylus vulgaris (see Large Strongyles in Horses).
Natural Openings in the Abdomen
There are several natural openings or spaces within the abdominal cavity that can be important in conditions causing colic. The inguinal canal provides an opening through which intestine might pass and become trapped. Although inguinal hernias are common in young foals, they rarely cause clinical problems; the situation is considerably different in stallions. Similarly, if the ventral abdominal wall fails to form properly around the umbilicus, an opening remains and the potential exists for intestinal problems to develop secondary to an umbilical hernia. The epiploic foramen, a natural opening between the portal vein, the caudal vena cava, and the caudate lobe of the liver, can be the site of intestinal incarcerations. Finally, there is a natural space between the dorsal aspect of the spleen and the left kidney. This space is bounded by the renosplenic ligament, a strong band of tissue that connects the dorsomedial aspect of the spleen with the fibrous capsule of the left kidney. This ligament provides a “shelf” over which large colon can be displaced.
Colonic Motility Patterns
Normograde peristalsis in the left ventral colon moves ingesta toward the left dorsal colon, and the muscles in the wall of the left dorsal colon contract to move the ingesta toward the diaphragmatic flexure. There is evidence, however, that the muscles in the left ventral colon contract in a retrograde fashion, from the pelvic flexure region toward the sternal flexure. Furthermore, these contractions appear to originate from a pacemaker region in the pelvic flexure. It has been hypothesized that this pacemaker senses either the size or the consistency of the feed particles in the ingesta and then initiates the appropriate motility pattern. If the ingesta has been digested sufficiently, it is moved in a normograde direction; if additional digestion is necessary, the ingesta is moved in a retrograde direction to retain it in the ventral colon. This theory has been proposed to help account for the common clinical occurrence of obstruction at or near the pelvic flexure.
Clinical Findings
Numerous clinical signs are associated with colic. The most common include pawing repeatedly with a front foot, looking back at the flank region, curling the upper lip and arching the neck, repeatedly raising a rear leg or kicking at the abdomen, lying down, rolling from side to side, sweating, stretching out as if to urinate, straining to defecate, distention of the abdomen, loss of appetite, depression, and decreased number of bowel movements. It is uncommon for a horse with colic to exhibit all of these signs. Although they are reliable indicators of abdominal pain, the particular signs do not indicate which portion of the GI tract is involved or whether surgery will be needed.
Diagnosis
A diagnosis can be made and appropriate treatment begun only after thoroughly examining the horse, considering the history of any previous problems or treatments, determining which part of the intestinal tract is involved, and identifying the cause of the particular episode of colic. In most instances, colic develops for one of four reasons: 1) The wall of the intestine is stretched excessively by either gas, fluid, or ingesta. This stimulates the stretch-sensitive nerve endings located within the intestinal wall, and pain impulses are transmitted to the brain. 2) Pain develops due to excessive tension on the mesentery, as might occur with an intestinal displacement. 3) Ischemia develops, most often as a result of incarceration or severe twisting of the intestine. 4) Inflammation develops and may involve either the entire intestinal wall (enteritis) or the covering of the intestine (peritonitis). Under such circumstances, proinflammatory mediators in the wall of the intestine decrease the threshold for painful stimuli.
The list of possible conditions that cause colic is long, and it is reasonable first to determine the most likely type of disease and begin appropriate treatments and then to make a more specific diagnosis, if possible. The general types of disease that cause colic include excessive gas in the intestinal lumen (flatulent colic), simple obstruction of the intestinal lumen, obstruction of both the intestinal lumen and the blood supply to the intestine (strangulating obstruction), interruption of the blood supply to the intestine alone (nonstrangulating infarction), inflammation of the intestine (enteritis), inflammation of the lining of the abdominal cavity (peritonitis), erosion of the intestinal lining (ulceration), and “unexplained colic.” In general, horses with strangulating obstructions and complete obstructions require emergency abdominal surgery, whereas horses with the other types of disease can be treated medically.
The history of the present colic episode and previous episodes, if any, must be considered to determine whether the horse has had repeated or similar problems or whether this episode is an isolated event. The duration of the present episode, the rate of deterioration of the horse's cardiovascular status, the severity of pain, whether feces have been passed, and the response to any treatments are important pieces of information. It is also critical to determine the horse’s deworming history (schedule, treatment dates, drugs used), when the teeth were floated last, if any changes in feed or water supply or amount have occurred, whether or not the horse is a "cribber," and whether the horse was at rest or exercising when the colic episode started.
The physical examination should include assessment of the cardiopulmonary and GI systems. The oral mucous membranes should be evaluated for color, moistness, and capillary refill time. The mucous membranes may become cyanotic or pale in horses with acute cardiovascular compromise and eventually hyperemic or muddy as peripheral vasodilation develops later in shock. The capillary refill time (normal ~1.5 sec) may be shortened early but usually becomes prolonged as vascular stasis (venous pooling) develops. The membranes become dry as the horse becomes dehydrated. The heart rate increases due to pain, hemoconcentration, and hypotension; therefore, higher heart rates have been associated with more severe intestinal problems (strangulating obstruction). However, it is important to note that not all conditions requiring surgery are accompanied by a high heart rate.
An important aspect of the physical examination is the response to passing a nasogastric tube. Because horses can neither regurgitate nor vomit, adynamic ileus, obstructions involving the small intestine, or distention of the stomach with gas or fluid may result in gastric rupture. Passing a stomach tube may, therefore, save the horse’s life and assist in diagnosis of these conditions. If fluid reflux occurs, the volume and color of the fluid should be noted. In healthy horses, it is common to retrieve <1 L of fluid from the stomach.
The abdomen and thorax should be auscultated and the abdomen percussed. The abdomen should be auscultated over several areas (cecum on the right, small intestine high on the left, colon lower on both the right and left). Intestinal sounds associated with episodes of pain may indicate an intraluminal obstruction (eg, impaction, enterolith). Gas sounds may indicate ileus or distention of a viscus. Fluid sounds may indicate impending diarrhea associated with colitis. A complete lack of sounds is usually associated with adynamic ileus or ischemia. Percussion helps identify a grossly distended segment of intestine (cecum on right, colon on left) that may need to be trocarized. The respiratory rate may be increased due to fever, pain, acidosis, or an underlying respiratory problem. Diaphragmatic hernia is also a possible cause of colic.
The most definitive part of the examination is the rectal examination. The veterinarian should develop a consistent method of palpating for the following: aorta, cranial mesenteric artery, cecal base and ventral cecal band, bladder, peritoneal surface, inguinal rings in stallions and geldings or the ovaries and uterus in mares, pelvic flexure, spleen, and left kidney. The intestine should be palpated for size, consistency of contents (gas, fluid, or impacted ingesta), distention, edematous walls, and pain on palpation. In healthy horses, the small intestine cannot be palpated; with small-intestinal obstruction, strangulating obstruction, or enteritis, the distended duodenum can be palpated dorsal to the base of the cecum on the right side of the abdomen, and distended loops of jejunum can be identified in the middle of the abdomen.
A sample of peritoneal fluid (obtained via paracentesis performed aseptically on midline) often reflects the degree of intestinal damage. The color, cell count and differential, and total protein concentration should be evaluated. Normal peritoneal fluid is clear to yellow, contains <5,000 WBCs/μL (most of which are mononuclear cells), and <2.5 g of protein/dL.
The age of the horse is important, because a number of age-related conditions cause colic. The more common of these include the following: in foals—atresia coli, meconium retention, uroperitoneum, and gastroduodenal ulcers; in yearlings—ascarid impaction; in the young—small-intestinal intussusception, nonstrangulating infarction, and foreign body obstruction; in the middle-aged—cecal impaction, enteroliths, and large-colon volvulus; and in the aged—pedunculated lipoma and mesocolic rupture.
Ultrasonographic evaluation of the abdomen may help differentiate between diseases that can be treated medically and those that require surgery. The technique also can be applied transrectally to clarify findings noted on rectal palpation. In foals, echoes from the large colon and small intestine are commonly identified from the ventral abdominal wall, whereas only large-colon echoes are usually seen in adult horses. The large colon can be identified by its sacculated appearance. The duodenum can be identified in the tenth intercostal space and traced around the caudal aspect of the right kidney. The jejunum is rarely identified during transabdominal ultrasonographic examination of normal adult horses, whereas the thick-walled ileum can be identified by transrectal examination.
The most common abnormalities identified by ultrasonography include inguinal hernia, renosplenic entrapment of the large colon, sand colic, intussusception, enterocolitis, right dorsal colitis, and peritonitis. Stallions with inguinal hernia have incarcerated intestine on the affected side; it is possible to identify the intestine and to obtain information concerning the thickness of its wall as well as the presence or lack of peristalsis. In horses with renosplenic entrapment of the large colon, the tail of the spleen or the left kidney cannot be imaged, or the gas-filled large colon is present in the caudodorsal aspect of the abdomen in the region of the renosplenic space. Horses with sand colic have granular hyperechoic echoes originating from the affected portion of the colon. The characteristic finding in horses with intussusception is the “bull’s eye” appearance of the affected portion of the small intestine. Very often the intestine proximal to the intussusception is distended, and the strangulated portion is thickened. Horses with enterocolitis frequently have evidence of hyperperistalsis, thickened areas of the bowel wall, and fluid distention of the intestine. In contrast, horses with right dorsal colitis commonly have marked thickening of the wall of the right dorsal colon. In horses with peritonitis, the peritoneal fluid may be anechoic, or there may be evidence of flocculent material and fibrin between serosal surfaces of the viscera.
Treatment
Horses with colic may need either medical or surgical treatments. Almost all require some form of medical treatment, but only those with certain mechanical obstructions of the intestine need surgery. The type of medical treatment is determined by the cause of colic and the severity of the disease. In some instances, the horse may be treated medically first and the response evaluated; this is particularly appropriate if the horse is mildly painful and the cardiovascular system is functioning normally. Ultrasonography can be used to evaluate the effectiveness of nonsurgical treatment. If necessary, surgery can be used for diagnosis as well as treatment.
If evidence of intestinal obstruction with dry ingesta is found on rectal examination, a primary aim of treatment is to rehydrate and evacuate the intestinal contents. If the horse is severely painful and has clinical signs indicating loss of fluid from the bloodstream (high heart rate, prolonged capillary refill time, and discoloration of the mucous membranes), the initial aims of treatment are to relieve pain, restore tissue perfusion, and correct any abnormalities in the composition of the blood and body fluids (see Table: General Concepts Regarding Fluid Needs in Dehydrated Horses). If damage to the intestinal wall (as a result of either severe inflammation or a displacement or strangulating obstruction) is suspected, steps should be taken to prevent or counteract the ill effects of bacterial endotoxins that cross the damaged intestinal wall and enter the bloodstream. Finally, if there is evidence the colic episode is caused by parasites, one aim of treatment is to eliminate the parasites.
General Concepts Regarding Fluid Needs in Dehydrated Horses
Pain Relief:
In most cases of colic, pain is mild, and analgesia is all that is needed. In these instances, the cause of colic is presumed to be spasm of intestinal muscle or excessive gas in a portion of the intestine. If, however, the pain is due to an intestinal twist or displacement, some of the stronger analgesics may mask the clinical signs that would be useful in making a diagnosis. For these reasons, a thorough physical examination should be completed before any medications are given. However, because horses with severe colic or pain may hurt themselves and become dangerous to people nearby, analgesics often must be given first. Additionally, many horses with less severe problems may need pain relief until the other treatments have time to be effective. An analgesic that has the fewest adverse effects and causes the least alteration in the horse’s attitude should be selected.
Medications used commonly for abdominal pain are NSAIDs that reduce the production of prostaglandins. When these drugs are used as recommended, their toxic effects on the kidneys and GI tract occur infrequently. Clinical experience suggests that flunixin meglumine may mask the early signs of conditions that require surgery and, therefore, must be used carefully in horses with colic.
The most commonly used sedative for colic is xylazine, an α2-agonist. Within a few minutes after administration, the horse stands quietly and is less responsive to pain. Unfortunately, the effects of xylazine are short-lived, and it inhibits intestinal muscular activity; it also decreases cardiac output and thus reduces blood flow to the tissues. Detomidine, a more potent α2-agonist that is much longer acting, is used successfully in similar circumstances.
Of the narcotic analgesics, butorphanol is used most often in horses with colic. Butorphanol has few adverse effects on the GI tract or heart. However, when given in large doses, narcotics can cause excitement, and the horse may become unstable. Butorphanol is frequently combined with an α2-agonist to produce a more prolonged period of analgesia.
Although pain relief usually is provided by analgesics, there are other important ways to reduce the degree of pain. For example, passing a nasogastric tube (also an important part of the diagnostic evaluation) may remove any fluid that has accumulated in the stomach because of an obstruction of the small intestine. The removal of this fluid not only relieves pain from gastric distention but also prevents rupture of the stomach.
Horses with displacement of the colon over the renosplenic ligament (ie, left dorsal displacement of the colon) may benefit from administration of phenylephrine. This drug is given to contract the spleen and often is followed by light exercise on a lunge line in an effort to dislodge the entrapped colon. It is important to note that this treatment may cause fatal hemorrhage due to hypertension in horses >15 yr old.
Fluid Therapy:
Many horses with colic benefit from fluid therapy to prevent dehydration and maintain blood supply to the kidneys and other vital organs. The fluids may be given either through the nasogastric tube or IV, depending on the particular intestinal problem (see General Concepts Regarding Fluid Needs in Dehydrated Horses). Horses with strangulating obstruction or enteritis must be given fluids IV, because absorption of fluids from the diseased intestine is impaired and fluid may be secreted into the lumen of the intestine. The latter mechanism causes a buildup of fluid in the intestine, which must be removed from the stomach through a nasogastric tube. This abnormal movement of body fluids into the intestine contributes to the development of circulatory shock, which is often the ultimate cause of death.
In healthy horses, most of the fluid in the intestinal tract is reabsorbed in the cecum and colons. In fact, ~95% of the fluid that normally enters the lumen of the large intestine is returned to the bloodstream. Therefore, horses with intestinal obstructions near the pelvic flexure usually require relatively small amounts of IV fluids, whereas horses with small-intestinal obstructions need extremely large amounts.
The volume and type of fluid to be given are determined by the severity and cause of the problem. Laboratory tests to determine the degree of hemoconcentration and whether concentrations of electrolytes are abnormal are critical for accurate treatment of horses with severe colic. The balance of body fluids can be reestablished by administering IV fluids formulated to replenish the deficient electrolyte(s). In most instances, however, fluid therapy must be started before laboratory results are available, particularly when the horse is showing clinical signs of circulatory shock.
When IV fluids are needed but the clinical signs are mild to moderate, the horse is usually given 8–10 L of a sterile replacement fluid that contains electrolytes in concentrations similar to those that normally exist in the blood. This volume is administered throughout 1–2 hr, and the horse is reevaluated to determine whether additional fluids are needed. Horses in circulatory shock require much larger volumes of IV fluids, given as rapidly as possible; as much as 20 L in 1 hr may be needed to reestablish tissue perfusion. In severe cases, hypertonic saline (7% NaCl) may be given to rapidly increase plasma volume. Depending on the cause of colic, IV fluids may be needed for several days until intestinal function has returned, electrolyte concentrations are balanced, and the horse can maintain its fluid needs by drinking. Under such circumstances, the daily IV fluid requirements may range from 30 to 100 L.
Fluids are sometimes given through the nasogastric tube as part of the treatment of impactions of the colon. Many clinicians believe the same result can be accomplished by giving large volumes of fluids IV. If the horse will not drink voluntarily and there is no obstruction in the small intestine, hydration may be maintained by administering fluids through the tube. Fluids or medications should not be given through the nasogastric tube if fluid reflux is being removed from the stomach, because this indicates either the stomach or the small intestine is not emptying properly.
Protection Against Components of Enteric Bacteria:
In healthy horses, the mucosal lining of the GI tract restricts enteric bacteria and their structural components (eg, endotoxins, lipoproteins, nucleic acids, flagellin) to the intestinal lumen. These bacterial components exist in high concentrations in the intestinal lumen, because they are released when the bacteria die or, in some cases, when bacteria multiply rapidly. However, when this mucosal barrier is disrupted, as occurs with intestinal ischemia or inflammation, the bacterial components can move into the peritoneal cavity and then be absorbed into the systemic circulation. Based on recent research studies, equine leukocytes are most sensitive to endotoxins but also respond strongly to other components, most notably flagellin. Most studies performed to date have focused on endotoxins, because they are assumed to be the primary triggers for the systemic inflammatory responses that occur in many horses with GI disease. These responses can include fever, depression, hypotension, reduced tissue perfusion, and coagulation abnormalities. In fact, many equine practitioners refer to these physiologic responses as “endotoxemia.” Thus, minimizing the inflammatory responses to endotoxemia is a vital part of colic therapy.
Prostaglandins are involved in causing many of endotoxin’s early ill effects. Flunixin meglumine reduces the cellular production of prostaglandins and can help prevent some of their effects. Because flunixin can help prevent some of the early effects of endotoxemia at dosages less than the recommended dosage (1.1 mg/kg), smaller dosages (0.25 mg/kg) can be administered without masking clinical signs associated with conditions that require surgery.
There is considerable controversy regarding the efficacy of plasma or serum that contains antibodies designed to neutralize endotoxin. These antibodies are directed against the components of endotoxins that are consistent among different gram-negative bacteria. The results of clinical studies using such antibodies have been conflicting, with evidence of protection being seen in some studies and no positive effects identified in others. This apparent lack of efficacy of anti-endotoxin antibodies also may indicate that some of the systemic inflammatory responses encountered are triggered by other bacterial components. Because endotoxin itself stimulates the generation of a wide array of inflammatory substances that ultimately produce the pathophysiologic effects, neutralizing antibodies should be used as early as possible in the course of the disease.
As an alternative approach, polymyxin B has been used to prevent endotoxin from interacting with the horse’s inflammatory cells. Polymyxin B has well-documented nephrotoxicity; however, concentrations of polymyxin B that bind endotoxin are far less than those that cause toxic effects. Polymyxin B has been evaluated in several experimental studies of endotoxemia and is being used in clinical cases at 1,000–5,000 U/kg, bid-tid. This form of therapy should be started as early as possible in the clinical course of the disease. In addition, fluid replacement therapy should be maintained in hypovolemic horses, and serum creatinine concentration should be closely monitored. This latter concern is especially relevant for azotemic neonatal foals, because they appear to be more susceptible to the nephrotoxic adverse effects of polymyxin B.
Intestinal Lubricants and Laxatives:
A common cause of colic in horses is simple obstruction of the large colon by dehydrated ingesta, sometimes mixed with sand. These impactions generally develop near the pelvic flexure or in the right dorsal colon but may involve any portion of the large colon, descending colon, or cecum. In most instances, lubricants or fecal-softening agents given through a nasogastric tube soften the impacted ingesta, allowing it to be passed. This form of therapy can be aided by the simultaneous administration of IV fluids. Keeping the horse muzzled is advised to prevent further impaction of feed material while the obstruction is softening.
Mineral oil is the most commonly used medication in the treatment of a large-colon impaction. It coats the inside of the intestine and aids the normal movement of ingesta along the GI tract. It is administered through a nasogastric tube, as much as 4 L, once or twice daily, until the impaction is resolved. Although mineral oil is safe, it is not highly effective in treating severe impactions or sand impactions, because it may simply pass by the obstruction without softening it.
Dioctyl sodium sulfosuccinate (DSS) is a soap-like compound that acts by drawing water into the dry ingesta. It is more effective than mineral oil in softening impactions; however, it may interfere with the normal fluid absorptive functions of the colon and can be toxic. Thus, DSS can be given safely only in small quantities two times 48 hr apart.
A safe and useful compound to treat impactions, especially those containing sand, is psyllium hydrophilic mucilloid. When mixed with water, it forms a gelatinous mass that carries ingesta along the GI tract. Although usually given through a nasogastric tube to horses with impactions, psyllium also may be used as a preventive by mixing the dry powder into the feed. Horses that live in a sandy environment or that persistently develop impactions may be given psyllium powder, 400 g/500 kg/day, in their feed for 7 days. This treatment is repeated 2–3 times each year in an effort to prevent development of sand impactions.
Strong laxatives that stimulate intestinal contractions are not commonly used to treat impactions and, in fact, may worsen the problem. Occasionally, horses with extremely hard impactions are treated with magnesium sulfate, which draws body fluids into the GI tract. Adverse effects include dehydration and an increased risk of diarrhea.
Fluid therapy, whether the fluids are administered through a nasogastric tube or IV, is an important and effective part of treating horses with colonic or cecal impactions. If an impaction does not start to break down within 3–5 days, surgery may be necessary to evacuate the intestine and help restore normal motility.
Larvicidal Deworming:
The normal migratory routes of the larvae of large bloodworms, particularly Strongylus vulgaris, have been implicated in many cases of colic. In response to the migratory and maturation processes of the larvae in the cranial mesenteric artery, the wall of the artery becomes thickened and forms loose plaques of inflammatory tissue. It has been hypothesized that these plaques activate coagulation, resulting in thromboembolism. The blood supply to the intestine may be reduced, resulting in altered intestinal motility, a change in the absorption of nutrients from the intestine, or death of the intestine. Thus, thromboembolism has been presumed to be a cause of recurrent episodes of colic and weight loss.
Modern deworming medications, such as ivermectin and moxidectin, have activity against migrating S vulgaris larvae. Fenbendazole kills migrating strongyles if given at twice the recommended dosage daily for 5 days or at 10 times the recommended dosage daily for 3 days. As a result of common use of these anthelmintics, chronic intermittent colic once thought to be caused by thromboembolism or parasite larval migration has largely been eliminated from equine practice.
There is considerable evidence that damage caused by cyathostomins causes colic, diarrhea, and loss of condition, particularly in young horses. These signs are seen on a seasonal basis and are synchronous with the emergence of large numbers of encysted larvae into the lumen of the large colon. In temperate areas of the Northern hemisphere, the larvae encyst during the winter months and emerge in the late winter and spring, causing ulceration, edema, and inflammation of the mucosa of the large colon. This may result in diarrhea, protein loss, weight loss, and mild intermittent colic and fever. Horses with cyathostomosis require treatment with larvacidal dosages of anthelmintics such as ivermectin, moxidectin, and fenbendazole. Some horses require analgesics, supportive care, and proper nutritional support.
Also see Gastrointestinal Parasites of Horses for a detailed discussion of treatment for large and small strongyles.
Surgery:
Surgery usually is necessary if there is a mechanical obstruction to the normal flow of ingesta that cannot be corrected medically or if the obstruction also interferes with the intestinal blood supply. The latter conditions result in death of the horse unless surgery is performed quickly. Occasionally, surgery is indicated as an exploratory diagnostic procedure for horses with chronic colic that have not responded to routine medical therapy.
Under most circumstances, horses exhibiting signs of severe abdominal pain nonresponsive to analgesic therapy require emergency abdominal surgery. Generally, the lumen of the intestine is completely obstructed, such as occurs with a strangulating obstruction, enterolithiasis, or severe displacement. Similarly, horses with an abnormally distended intestine on rectal examination and peritoneal fluid with an increased total protein concentration and number of erythrocytes probably have a strangulating lesion that requires surgical correction. However, these classic findings that characterize horses requiring emergency surgery do not always exist. Some horses with mild or moderate pain may also require surgery, and a judgment must be based on a thorough physical examination and other methods of evaluation, including abdominal ultrasonography. Some of the more commonly used indications for surgery in horses with colic include uncontrollable pain; >4 L of fluid reflux from the stomach; no borborygmi on auscultation; peritoneal fluid with increased protein, erythrocytes, and toxic neutrophils; and a tightly distended intestine, displaced colon, or enterolith or foreign body identified on rectal examination.
Performing surgery (if indicated) early is critical to success and improves the prognosis for survival. Therefore, it is more important to decide whether the horse should be referred to a clinic where surgery could be performed if needed than to determine whether emergency surgery is definitively required. It is generally prudent to refer the following types of cases: 1) a horse that responds initially to an analgesic but requires additional analgesic therapy a few hours later, 2) a horse that continues to exhibit signs of pain despite administration of analgesics, 3) a horse that remains painful but has normal peritoneal fluid, 4) a horse with distended loops of small intestine on rectal examination, or 5) a horse with large quantities of fluid removed from the stomach but no distended small intestine palpable on rectal examination.
When surgery is required, in most instances, the horse is anesthetized and positioned in dorsal recumbency, and the surgical incision is made on the ventral midline. Once the peritoneal cavity is entered, portions of the intestine should be examined to determine the definitive cause of the colic. Correction may involve repositioning a displaced portion of intestine, removing an obstruction, or resecting devitalized intestine. When devitalized segments of intestine must be removed or an enterotomy performed, postoperative care may include antibiotics, IV fluids, polymyxin B, antibodies directed against endotoxin, and NSAIDs to combat endotoxemia. When a displaced segment of intestine is simply returned to its normal location, the postoperative care is much less intense. Each horse must be handled individually, and its treatment needs are based on the response to surgery and development of complications.
Prognosis
A large retrospective study in the USA documented an overall survival rate of 60% for horses with colic and a survival rate of 50% for those horses undergoing abdominal surgery, including those euthanized during surgery for inoperative conditions. Survival rates for horses with strangulating obstruction and inflammatory diseases were only 24% and 42%, respectively. In contrast, horses with an undefined cause for the colic episode had a survival rate of 94%. When the segment of the GI tract was considered, the survival rates for conditions affecting the small intestine and stomach were poorer than for those affecting the large colon. In addition, conditions that interfered with both the passage of ingesta and the intestinal blood supply dramatically decreased the chances of survival. The results of more recent studies are far more promising, with survival rates for horses undergoing emergency abdominal surgery often >80%. Furthermore, there have been reports documenting survival rates of 70% for horses requiring resection of strangulated small intestine or correction of large-colon volvulus. In earlier retrospective studies, these conditions were associated with survival rates ≤30%. Although data on longterm survival (ie, the horse returning to its intended use) are more difficult to obtain, recent findings indicate that most horses that die or are euthanized because of serious problems do so within 3 mo after surgery.
Values obtained from several variables are often combined to predict survival in horses with colic. Prognostic indicators include pain assessment, intestinal distention, mucous membrane color, and cardiovascular system function. Survival rates are highest for horses with mild abdominal pain and are lowest for horses with severe pain. Horses with palpable intestinal distention have lower survival rates than horses lacking evidence of intestinal distention, and survival rates are even lower if no intestinal sounds are audible on auscultation of the abdomen. Red mucous membranes are frequently associated with endotoxemia, which decreases the survival rate. Cardiovascular system function reflects the degree of shock and, therefore, correlates with the prognosis for survival. For instance, horses with low systolic blood pressure or a high heart rate have a decreased chance of survival.
Of the laboratory analyses used to predict survival, blood lactate concentration and the anion gap are used most often. Measurement of blood lactate has been used as an indicator of tissue perfusion, with increasing concentrations of lactic acid corresponding with poor tissue perfusion. In recent studies, changes in blood lactate concentration over time have been particularly useful to determine the prognosis for survival, with increasing concentrations being associated with a poor prognosis. Furthermore, changes in peritoneal fluid lactate concentrations over time have been used to help identify horses that require emergency abdominal surgery. Similarly, the anion gap (the calculated difference between the measured cations and the measured anions) reflects the generation of organic anions, most notably lactic acid, due to reduced tissue perfusion. The concentration of protein in the peritoneal fluid also has been used to predict survival, with higher concentrations associated with a poorer prognosis.
Resources In This Article
- Colic in Horses
- Overview of Colic in Horses
- Diseases Associated with Colic by Anatomic Location