Mycotic Diseases of Marine Mammals
- Marine Mammals
- Overview of Marine Mammals
- Management of Marine Mammals
- Environmental Diseases of Marine Mammals
- Nutrition and Nutritional Diseases of Marine Mammals
- Bacterial Diseases of Marine Mammals
- Mycotic Diseases of Marine Mammals
- Parasitic Diseases of Marine Mammals
- Viral Diseases of Marine Mammals
- Neoplastic Diseases of Marine Mammals
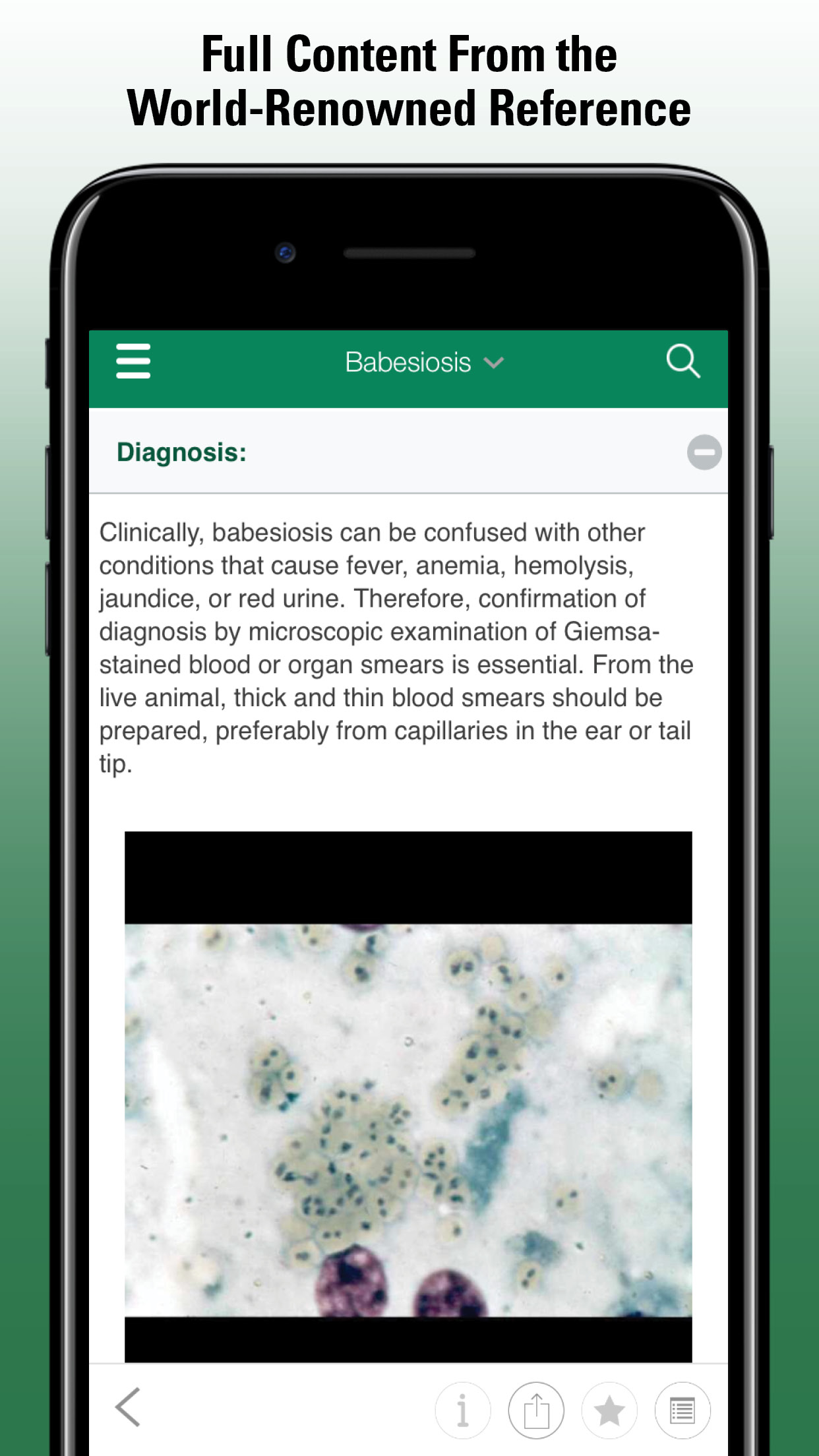
Captive marine mammals seem particularly prone to fungal infections (see Fungal Infections). Most appear to be secondary to stress, environmental compromise, or other infectious disease. Some systemic mycoses have distinct geographic distributions. Diagnosis is based on clinical signs and confirmed by identification of the organism in biopsy or, preferably, culture. Wet mounts in lactophenol or cotton blue may render an immediate diagnosis with some of the morphologically distinct fungi. Tissue smears cleared in warm 10% potassium hydroxide can be examined to identify characteristic fruiting bodies or hyphae.
Topical medication of pinnipeds for dermatophytosis is feasible. Smaller cetaceans can be kept out of water in a sling for 2–24 hr, if areas of the body not being treated are kept moist. Otherwise, systemic therapy is used.
Aspergillosis:
Fatal pulmonary aspergillosis has been diagnosed in several species of cetaceans, including bottlenose dolphins and killer whales, and in several pinnipeds, including Antarctic fur seals (Arctocephalus gazella), harbor seals, and California sea lions. In addition to disseminated aspergillosis, case reports of aspergillomas of the inner ear have been reported in free-ranging harbour porpoise, and cutaneous aspergillosis has been seen in gray seals (Halichoerus grypus) with concomitant mycobacteriosis. The respiratory form has been a postmortem diagnosis. Cutaneous lesions have responded to topical povidone-iodine with ketoconazole therapy (10 mg/kg/day, PO).
Candidiasis:
This common mycotic disease in captive cetaceans occurs secondary to stress, unbalanced water disinfection with chlorines, or indiscriminate antibiotic therapy. Candidiasis is also reported in pinnipeds and has been found in cultures of wild cetacean blow holes and stomachs. In clinical disease in cetaceans, the lesions usually are found around body orifices. At necropsy, esophageal ulcers are often found, particularly in the area of the gastroesophageal junction. In phocid pinnipeds, inflammation at the mucocutaneous junctions, particularly at the commissures of the mouth and around the eyes, anus, and vulva, is the common presentation. Diagnosis is based on identification of the yeast in cultures or biopsy. Candidiasis generally responds well to ketoconazole (6 mg/kg/day, PO) along with correction of any environmental deficits. Supplementation with prednisolone (0.01 mg/kg) may be appropriate to compensate for ketoconazole inhibition of glucocorticoid production. Fluconazole (2 mg/kg, bid) has also been used successfully. One anecdotal report suggests a possible toxic reaction to ketoconazole in a northern elephant seal (Mirounga angustirostris). Early detection and treatment is usually successful. Another opportunistic yeast, Cryptococcus neoformans, has been diagnosed in fatal advanced pulmonary disease in a bottlenose dolphin. Prolonged treatment with itraconazole (120 days) at routine mammalian doses was ineffective despite serum drug levels above the suggested therapeutic range.
Dermatophytosis:
Lobomycosis:
This disfiguring cutaneous disease is caused by infection with Lacazia loboi. The disease has been reported only in people and in Atlantic bottlenose Sotalia (Sotalia fluviatilis) dolphins. Culture of the organism has not been possible. Excisional therapy and systemic antifungal drugs have been used with varying success. Zoonotic transmission is strongly suspected in a case of a European handler of an infected dolphin. Most human cases have no history of marine mammal contact. Differential diagnoses include sporotrichosis, chromomycosis, paracoccidiodomycosis, and other fungal diseases characterized by extensive granuloma formation.
Systemic Mycoses:
The systemic mycoses of marine mammals are a zoonotic risk, and precautions should be taken to prevent infection when handling dead and diseased animals. Cystofilobasidiales has caused fatal disease in a California sea lion. Blastomycosis has caused fatal disease in bottlenose dolphins, California sea lions, a Stellar sea lion (Eumetopias jubatus), Northern fur seals, and polar bears. Fatal systemic histoplasmosis has been reported in a captive harp seal (Pagophilus groenlandicus), a bottlenose dolphin, and a Pacific white-sided dolphin. Coccidioidomycosis has been found in bottlenose dolphins, California sea lions, and sea otters. Blastomycosis has been successfully treated with intensive management, including 70 days of itraconazole (3.5 mg/kg/day, PO) combined with antibiotic and supportive therapy when indicated.
Zygomycetes:
Dermatologic conditions caused by various Fusarium spp have been reported in pygmy sperm whales (Kogia breviceps), Atlantic white-sided dolphins (Laegenorhynchus acutus), harbor seals, gray seals, California sea lions, and northern elephant seals. Diagnosis is based on culture or organism identification from biopsy. Cases have responded to ketoconazole (5 mg/kg/day for 10 days), fluconazole (0.5 mg/kg, bid for 21 days), or itraconazole (1 mg/kg/day for 120 days). Mucor spp and Entomophthora spp have caused fatal disease in bottlenose dolphins, harbour porpoises, and harp seals. Other zygomycetes have been diagnosed as a cause of fatal disseminated disease in various species of marine mammals. Localized fusariomycosis has been successfully treated in a captive beluga whale (Delphinapterus leucas) using voriconazole after surgical debridement. These infections should be considered diseases of debilitated animals; the underlying cause of the low host resistance to these opportunistic infections must be corrected if therapy is to be successful. Amphotericin B has been the therapy of choice for zygomycete infections, but newer imidazoles warrant consideration and may have clinical efficacy despite laboratory tests of resistance.
Resources In This Article
- Marine Mammals
- Overview of Marine Mammals
- Management of Marine Mammals
- Environmental Diseases of Marine Mammals
- Nutrition and Nutritional Diseases of Marine Mammals
- Bacterial Diseases of Marine Mammals
- Mycotic Diseases of Marine Mammals
- Parasitic Diseases of Marine Mammals
- Viral Diseases of Marine Mammals
- Neoplastic Diseases of Marine Mammals