Overview of Feline Infectious Peritonitis
- Feline Infectious Peritonitis
- Overview of Feline Infectious Peritonitis
Feline infectious peritonitis (FIP) is an immune-mediated disease triggered by infection with a feline coronavirus (FCoV). FCoV belongs to the family Coronaviridae, a group of enveloped, positive-stranded RNA viruses frequently found in cats. Coronavirus-specific antibodies are present in as many as 90% of cats in catteries and in as many as 50% of those in single-cat households. However, <5% of FCoV-infected cats develop FIP in multicat households.
Geographic Distribution:
FCoV infection and FIP occur worldwide with similar prevalence and are found in domestic and wild cats. FCoV strains can be classified into serotypes I and II, depending on their antigenetic relationship to canine coronavirus (CCV), and these subtypes vary in proportion among different countries. Among FCoV strains isolated in the field in the USA and Europe, 70%–95% are serotype I. In contrast, in Japan serotype II predominates. Most cats with FIP are infected with FCoV serotype I. However, both serotypes can cause FIP and both can cause clinically inapparent FCoV infections.
FCoV belongs to the same taxonomic cluster of coronaviruses as transmissible gastroenteritis virus, porcine respiratory coronavirus, CCV, and some human coronaviruses. In many species, coronaviruses have a relatively restricted organ tropism, mainly infecting the respiratory and/or GI tracts. In cats and mice, however, coronavirus infections can, in certain circumstances, involve multiple organs.
In addition to cats, other felid species are susceptible, and FCoV is also an important pathogen in nondomestic felids. There was evidence of FCoV infection in 195 of 342 nondomestic felids in southern Africa, which included both wild and captive animals. There is also a high incidence of FIP in nondomestic felids in captivity in the USA and Europe. Cheetahs in captivity seem to be highly prone to developing FIP; loss of genetic diversity and a genetic deficiency in their cellular immunity is thought to predispose them to the disease. Although FIP can only occur in felids, a clinically similar condition in ferrets is associated with ferret systemic coronavirus.
Etiology and Pathogenesis:
FIP is a sporadic disease thought to be caused by viral variants that develop within each specific cat. The pathogenesis of FIP is unclear, but there are two main hypotheses. The “internal mutation theory” states that cats are infected with the primarily avirulent FCoV that replicates in enterocytes; in some cats, a mutation occurs in a certain region of the FCoV genome that creates a new phenotype with the ability to replicate within macrophages. The presence of highly virulent strains of FCoV capable of consistently inducing FIP support this theory, albeit under experimental conditions. Several researchers speculate that some circulating feline enteric coronaviruses are closer to making critical mutations necessary for development of FIP, possibly explaining FIP outbreaks. No consistent mutation has yet been identified, although studies have suggested sequence differences in the spike protein, membrane protein, or NSP3c correlate with disease manifestation. Recent studies have found feline coronaviruses to have intact NSP3c genes, whereas most isolates from diseased tissues of FIP cases had disrupted NSP3c genes. Findings suggested that 3c-inactivated viruses only rarely replicate in the intestine, which possibly explains the rare incidence of FIP outbreaks. In additional work, it was concluded that mutation of the S1/S2 locus and modulation of a furin recognition site normally present in the S gene of enteric coronaviruses is a critical contributing factor for development of FIP.
The second hypothesis for the development of FIP is the existence of distinct circulating virulent and avirulent strains in a population, and exposure to the pathogenic strain, the viral load, and the cat’s immune response determine whether FIP will develop. It is likely both viral genetics and host immunity play a role. In both hypotheses, the key pathogenic event in the development of FIP is the massive replication of FCoV in macrophages. If the cat does not eliminate macrophages infected with replication-competent virus early in infection, the presence of the virus within circulating macrophages initiates an ultimately fatal arthus-type immune-mediated reaction, which defines FIP.
Factors that increase FCoV replication in the intestines (and increase the probability of the mutation) include young age, breed predisposition, immune status, stress, corticosteroid treatment, and surgery, as well as dosage and virulence of the virus and reinfection rate in multicat households. Crowded environments, such as catteries or shelters, also may increase stress and exposure to FCoV. Whenever FCoV infection exists, so does the potential for development of FIP.
After a cat becomes infected with FCoV via fecal-oral transmission (or less commonly, inhalation), the main site of viral replication is the intestinal epithelium. Replication of FCoV in the cytoplasm can destroy intestinal epithelial cells, leading to diarrhea in some cats. In many cats, infection persists for weeks to months in the absence of clinical signs. These cats shed FCoV either intermittently or continually and act as a source of infection for other cats. Previously, it was believed that avirulent FCoV remained confined to the digestive tract, did not cross the gut mucosa, and did not spread beyond the intestinal epithelium and regional lymph nodes. However, PCR can detect FCoV in the circulating macrophages of healthy cats from households with endemic FCoV, indicating that avirulent FCoV may also cause viremia.
FIP is an immune complex disease involving viral antigen, antiviral antibodies, and complement. Within weeks after macrophage invasion and replication, virions are found in the cecum, colon, intestinal lymph nodes, spleen, and liver after distribution by macrophages in the whole body, including the CNS. There are two possible explanations for the events that follow viral dissemination from the intestines. The first proposed mechanism is that FCoV-infected macrophages leave the bloodstream and carry virus into the tissues. The virus attracts antibodies, complement is fixed, and more macrophages and neutrophils are attracted to the lesion; as a consequence, typical pyogranulomatous changes develop. The alternative explanation is that FIP occurs as a result of circulating immune complexes lodging in blood vessel walls, fixing complement, and leading to development of the pyogranulomatous changes. It is assumed that these antigen–antibody complexes are recognized by macrophages but are not, as they should be, presented to killer cells, and thus are not destroyed.
In addition to virus, chemotactic substances, including complement and inflammatory mediators, are released from infected macrophages. Complement fixation leads to the release of vasoactive amines, which cause endothelial cell retraction and thus increased vascular permeability. Retraction of capillary endothelial cells allows exudation of plasma proteins and, hence, development of characteristic protein-rich exudates. Inflammatory mediators activate proteolytic enzymes that cause tissue damage.
Epidemiology and Transmission:
FCoV and FIP are a major problem in multicat households. The virus is endemic in environments in which many cats are kept together in a confined space (eg, catteries, shelters, pet stores). FCoV is found less commonly in free-roaming community cats, because they do not typically use the same locations to bury their feces; shared litter boxes are a major source of transmission in multicat households.
Although the prevalence of FCoV infection is very high in multicat households, <5% of cats in these situations develop FIP; the number is even lower in a single cat environment. The risk of developing FIP is higher for young and immunocompromised cats because the replication of FCoV in these animals is less controlled and, thus, the critical mutation is more likely to occur. More than half of the cats with FIP are <12 mo old. Sexually intact cats, males, and purebred cats have a higher incidence of FIP. Epidemiologic data suggest that the cat’s genetic background contributes to the manifestation of FIP. Investigators have described variation in breed resistance and susceptibility to FIP. Susceptibility to FIP is a polygenic inherited trait in Persians and Birmans. Breeds with higher prevalence of FIP include Abyssinian, Bengal, Birman, Himalayan, Ragdoll, and Rex.
FCoV is shed mainly in the feces. Infection is generally via the oronasal route. After natural infection, cats begin to shed virus in feces within 1 wk. In very early infection, it may be found in saliva, respiratory secretions, and urine. When naive cats in multicat households first encounter FCoV, it is likely that all will become infected (and develop antibodies); most will shed virus intermittently for a period of weeks or months. Some cats become chronic FCoV shedders, providing a continual source for reinfection of other cats. Cats that are antibody-negative are very unlikely to shed, whereas approximately one-third of all FCoV antibody-positive cats shed virus. Cats with high antibody titers are more likely to shed FCoV. They also are more likely to shed consistently and higher amounts of the virus. Most cats with FIP also shed non-mutated FCoV; however, the virus load in feces seems to decrease after a cat has developed FIP.
The major sources of FCoV for naive cats are litter boxes shared with shedding cats. Also, continuous reinfection through the contaminated litter box of a cat already infected seems to play an important role in the endemic survival of the virus. Rarely, virus can be transmitted through saliva, by mutual grooming, sharing the same food bowl, and through close contact. Sneezed droplet transmission is also rare but possible. It is uncertain whether FCoV transmission occurs to a significant degree at cat shows. Transmission by lice or fleas is considered unlikely. Transplacental transmission can occur but is very uncommon under natural circumstances. Most kittens that are removed from contact with virus-shedding adult cats at 5–6 wk of age do not become infected. Most commonly, kittens are infected at the age of 6–8 wk at a time when their maternal antibodies wane, mostly through contact with feces from their mothers or other FCoV-excreting cats.
FCoV is a relatively fragile virus that is destroyed by most household disinfectants and detergents. However, it may survive in cold or dry conditions (eg, in carpet) for as long as 7 wk outside the cat. Indirect fomite transmission is possible, and the virus can be transmitted for a short time via clothes, toys, and grooming tools.
Clinical Findings:
Feline Coronavirus Infection:
FCoV infection can cause a transient and clinically mild diarrhea and/or vomiting due to replication of FCoV in enterocytes. Kittens infected with FCoV may have a history of stunted growth or upper respiratory tract signs. Occasionally, the virus may cause severe diarrhea with weight loss, which may be unresponsive to treatment and continue for months. However, most FCoV-infected cats do not show clinical signs.
Feline Infectious Peritonitis:
Clinical signs of FIP vary depending on organ involvement. Many organs, including the liver, kidneys, pancreas, CNS, and eyes, can be involved. The clinical signs and pathologic findings are a consequence of the vasculitis and, less commonly, organ failure resultant from damage to the blood vessels that supply them. In all cats with nonspecific clinical signs, such as chronic weight loss or fever of unknown origin resistant to antibiotic treatment or recurrent in nature, FIP should be on the list of differential diagnoses.
The length of time between infection and development of clinical signs is unknown and depends on the immune response of the individual cat. Disease generally becomes apparent from a few weeks to 2 yr after the mutation has occurred. Cats are at greatest risk of developing FIP in the first 6–18 mo after infection with FCoV; the risk decreases to ~4% at 36 mo after infection.
Previously, three different forms of FIP were distinguished: 1) an effusive, exudative, “wet form”; 2) a noneffusive, nonexudative, granulomatous, parenchymatous “dry form”; and 3) a mixed form. The first form was characterized by a fibrinous peritonitis, pleuritis, and/or pericarditis with effusion in the abdomen, thorax, and/or pericardium, respectively. The second form was characterized by granulomatous changes in different organs that may include the eyes and CNS. Differentiation between these forms is not useful and is of value only for the diagnostic approach, because there is nearly always effusion to a greater or lesser degree in combination with more or less granulomatous changes present in cats with FIP. In addition, the forms can transform into each other. Cats with FIP may be alert or depressed. Some eat with a normal or even increased appetite; others are anorectic. Fever, weight loss, and/or icterus may be noted.
In cats with ascites, an abdominal swelling is commonly noticed. Fluctuation and a fluid wave may be present; in less severe cases, fluid can be palpated between the intestinal loops. Abdominal masses can sometimes be palpated, reflecting omental and visceral adhesions or enlarged mesenteric lymph nodes. Thoracic effusions may cause dyspnea, tachypnea, open-mouth breathing, or cyanotic mucous membranes. Auscultation reveals muffled heart sounds. In cats with pericardial effusions, heart sounds are muffled and typical changes can be seen on ECG and echocardiography. Effusions can be visualized by diagnostic imaging (eg, radiographs, ultrasound) and verified by a fluid centesis.
In cats without obvious effusion, in which mainly granulomatous changes are present, signs are often vague and include fever, weight loss, lethargy, and decreased appetite. If the lungs are involved, cats can be dyspneic, and thoracic radiographs may reveal patchy densities in the lungs. Abdominal palpation may reveal enlarged mesenteric lymph nodes and irregular kidneys or nodular irregularities in other viscera. Presenting clinical signs sometimes can be unusual. In some cats, abdominal tumors are suspected, but FIP is finally diagnosed at surgery or necropsy.
Cats with FIP frequently have ocular lesions. The most common ocular lesions are retinal changes, and a retinal examination should be performed in all cats with suspected FIP. FIP can cause cuffing of the retinal vasculature, which appears as fuzzy grayish lines on either side of the blood vessels. Occasionally, granulomatous changes are seen on the retina. Retinal hemorrhage or detachment may also occur. These changes, however, are not pathognomonic; similar changes can be seen in other systemic infectious diseases, including toxoplasmosis, systemic fungal infections, and feline immunodeficiency virus or feline leukemia virus infection. Uveitis is another common manifestation. Mild uveitis can manifest by color change of the iris. Uveitis may also manifest as aqueous flare, with cloudiness of the anterior chamber, which can be detected in a darkened room using focal illumination. Large numbers of inflammatory cells in the anterior chamber settle on the back of the cornea and cause keratic precipitates, which may be hidden by the nictitating membrane. Hemorrhage into the anterior chamber can occur. If aqueous humor is sampled, it may reveal increased protein and pleocytosis.
Neurologic signs are common in cats with FIP. These are variable and reflect the area of CNS involvement. The lesions are usually multifocal. The most common clinical sign is ataxia followed by nystagmus and seizures. In addition, incoordination, intention tremors, hyperesthesia, behavioral changes, and cranial nerve involvement can be seen. If cranial nerves are involved, neurologic signs such as visual deficits and loss of menace reflex may be present. When FIP lesions are located on a peripheral nerve or the spinal column, lameness, progressive ataxia, or paresis may be seen. Finding hydrocephalus on a CT scan is suggestive of neurologic FIP. In a study of 24 cats with FIP with neurologic involvement, 75% were found to have hydrocephalus on postmortem examination.
Solitary mural intestinal lesions have been described in cats with a histologic diagnosis of FIP. Diarrhea, vomiting, or obstruction can occur, and a suspected neoplastic mass can be found in the colon or ileocecocolic junction, with associated lymphadenopathy, and a markedly thickened and firm segment of bowel with multifocal pyogranulomas extending through the intestinal wall on histology.
Skin fragility syndrome was described in a cat with FIP, and other skin lesions (eg, nodular skin lesions, papular skin lesions, pododermatitis) may be present as well. Reproductive disorders, neonatal deaths, and fading kittens are not usually associated with FIP.
Lesions:
Histology of lesions is usually pathognomonic and is traditionally considered the gold standard for diagnosis of FIP. H&E-stained; samples typically contain localized perivascular mixed inflammation with macrophages, neutrophils, lymphocytes, and plasma cells. FCoV can be identified by immunohistochemistry in the macrophages within the lesions. Pyogranulomas may be large and consolidated, sometimes with focal tissue necrosis, or numerous and small. Lymphoid tissues in cats with FIP often show lymphoid depletion caused by apoptosis.
Diagnosis:
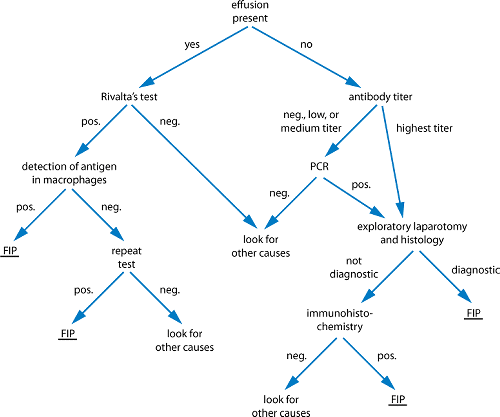
Reliable and rapid diagnosis of FIP is important but can be challenging. Difficulties arise from the lack of noninvasive confirmatory tests in cats without obvious effusion. Obtaining and analyzing effusion is minimally invasive and much more sensitive than diagnostic tests in blood. In cats with no effusion, several parameters including history, clinical signs, laboratory changes, and level of antibody titers should be considered to determine whether to use invasive confirmative diagnostic methods.
Hematology and Serum Biochemistry:
WBC counts can be decreased or increased. Lymphopenia is commonly present, mainly caused by apoptosis of uninfected T cells, primarily CD8+ T cells, as a result of high TNF-α concentrations produced by virus-infected macrophages. However, lymphopenia in combination with neutrophilia can occur in many severe diseases in cats. A mild to moderate nonregenerative anemia is another nonspecific finding that may be seen in almost any chronic disease in cats.
The most common laboratory abnormality in cats with FIP is an increase in total serum protein concentration caused by increased globulins, mainly γ-globulins, which occurs in >70% of cats with FIP. Total protein in cats with FIP can reach very high concentrations of ≥12 g/dL. The albumin to globulin ratio, however, has a significantly higher diagnostic value to distinguish FIP from other diseases than total serum protein or γ-globulin concentrations, because serum albumin also may decrease. Albumin loss in cats with FIP may be caused by glomerulonephritis secondary to immune complex deposition, by loss of protein due to exudative enteropathy in cases of granulomatous changes in the intestines, or by extravasation of protein-rich fluid during vasculitis. An optimal cut-off value of 0.8 was determined for the albumin to globulin ratio (specificity 82%, sensitivity 80%). If the serum albumin to globulin ratio is <0.8, the probability that the cat has FIP is high (92% positive predictive value [PPV]); if the albumin to globulin ratio is >0.8, the cat likely does not have FIP (61% negative predictive value [NPV]). Reference laboratories have variation in techniques for measurement of serum proteins. Serum protein electrophoresis may be performed in cats with suspected FIP to distinguish a polyclonal from a monoclonal hypergammaglobulinemia to differentiate FIP (and other chronic infection) from tumors such as multiple myelomas or other plasma cell tumors. However, hyperglobulinemia is generally nonspecific and reflects active inflammation.
Other laboratory parameters, including liver enzymes, bilirubin, urea (or BUN), and creatinine, can be variably increased depending on the degree and localization of organ damage but are not helpful in establishing an etiologic diagnosis. Hyperbilirubinemia and icterus are often seen in cats with FIP. High bilirubin (in the absence of hemolysis) and increased liver enzyme activity should raise the suspicion of FIP.
High serum levels (>3 mg/mL) of α-1-acid glycoprotein (AGP), a serum acute phase protein that is increased in cats with FIP, can support diagnosis, but levels also are increased in other inflammatory conditions; thus, these changes are not specific. Additionally, AGP may also be high in asymptomatic cats infected with FCoV, especially in households where FCoV is endemic. However, the most prominent increases in serum AGP concentration have been recorded in cats with FIP. Levels of AGP in serum and effusions increased 2- to 5-fold in cats with FIP, more than in diseases such as neoplasia and cardiomyopathy. In a study with a small number of cases, measurement of serum AGP concentrations was demonstrated to be most helpful, because it was the only diagnostic test in complete concordance with immunohistochemistry. Two factors must be considered when evaluating AGP concentration to support a clinical diagnosis of FIP: the magnitude of the increase in concentration and the pretest probability of FIP (compatible history and clinical findings). Serum amyloid A (SAA), another acute phase protein, increases 10-fold in the serum of cats with FIP compared with asymptomatic cats exposed to feline enteric coronaviruses. SAA may be a useful biomarker in the future.
Diagnostic Imaging:
A recent study highlighted specific concurrent ultrasonographic findings that should increase the index of suspicion for FIP, including abdominal lymphadenopathy, peritoneal or retroperitoneal effusion, renomegaly, irregular renal contour, hypoechoic subcapsular echogenicity, and diffuse changes within the intestines. In most cats in the study population, the liver and spleen were normal in echogenicity. A normal abdominal ultrasound does not exclude the possibility of FIP infection.
Effusion Fluid:
Tests on effusion have a much higher diagnostic value than tests performed on blood. Fluid can be obtained through ultrasound-guided fine-needle aspiration or by using the “flying cat technique” in case of ascites. Although clear yellow effusions of sticky consistency are considered typical, the presence of this type of fluid in body cavities alone is not diagnostic. Cases with pure chylous effusion have been reported. Usually, the protein content is very high (>3.5 g/dL), consistent with an exudate, whereas the cellular content is low (<5,000 nucleated cells/mL), resembling a modified transudate or even pure transudate. Major differential diagnoses for these effusions include inflammatory liver disease, lymphoma, heart failure, and bacterial peritonitis or pleuritis. LDH activity typically is high (>300 IU/L). Cytology is variable but often consists predominantly of macrophages and nondegenerate neutrophils (in much lower numbers than seen with bacterial infection). These effusions can usually be differentiated from bacterial infection or lymphoma by the presence of malignant cells, degenerate neutrophils, or intracellular bacteria on cytology and bacterial growth on culture, respectively. The albumin to globulin ratio of the effusion can be measured: a ratio of <0.5 is strongly correlated with FIP, with a PPV between 66% and 95%, depending on the prevalence of FIP in the cat’s environment. An albumin to globulin ratio >0.81 has a 100% NPV, essentially excluding FIP.
Rivalta’s test is a simple, inexpensive method that does not require special laboratory equipment and can be performed easily in private practice. It is very useful in cats to differentiate between effusions caused by FIP and effusions caused by other diseases. The high protein content and high concentrations of fibrin and inflammatory mediators lead to a positive reaction. To perform the test, a transparent reagent tube (10 mL) is filled with ~8 mL distilled water, to which 1 drop of acetic acid (highly concentrated vinegar, 98%) is added and mixed thoroughly. On the surface of this solution, 1 drop of the effusion fluid is carefully layered. If the drop disappears and the solution remains clear, the Rivalta’s test is defined as negative. If the drop retains its shape, stays attached to the surface, or slowly floats down to the bottom of the tube (drop- or jelly-fish-like), the test is defined as positive. Rivalta’s test has a high PPV (86%) and a very high NPV (96%) for FIP. Positive results can sometimes be seen in cats with bacterial peritonitis or lymphoma. Those effusions, however, are usually easy to differentiate through macroscopic examination, cytology, and bacterial culture.
Cerebrospinal Fluid:
Analysis of cerebrospinal fluid (CSF) from cats with neurologic signs due to FIP lesions may reveal increased protein (50–350 mg/dL with a normal value of <25 mg/dL) and pleocytosis (100–10,000 nucleated cells/mL) containing mainly neutrophils, lymphocytes, and macrophages (a relatively nonspecific finding). Many cats with neurologic signs caused by FIP have normal CSF. In one study, typical CSF findings in cats with FIP were a protein concentration >200 mg/dL and a WBC count of >100 cells/μL, which consisted predominantly of neutrophils.
Measurement of Antibodies:
There is no FIP-specific antibody test; all that can be measured is antibodies against FCoV. Antibody titers measured in serum are extensively used as a diagnostic tool. However, most FCoV antibody-positive cats never develop FIP. Thus, antibody titers must be interpreted extremely cautiously and should never be used as the sole test to diagnose FIP. Antibody testing still has a certain role in the diagnosis and, more importantly, in the management of multicat households, when done by appropriate methodologies and when results are properly interpreted. However, antibody testing can only be useful if the laboratory is reliable and consistent. Low or medium titers have no diagnostic value. If interpreted carefully, however, very high titers can be of certain diagnostic value. A very high titer in a cat with compatible clinical signs (1:1,600) has a 94% PPV for FIP; a negative titer has a 90% NPV for FIP. Cats with high antibody titers are more likely to shed FCoV and to shed more consistently higher amounts of the virus. Thus, the titer is directly correlated with virus replication rate and the amount of virus in the intestines. Screening a cattery for the presence of FCoV or screening a cat before introduction into an FCoV-free cattery are additional indications.
Measuring antibodies in fluids (eg, effusion, CSF) other than blood has been investigated. Detection of anticoronavirus antibodies in effusion fluid has a PPV of 90% and a NPV of 29% for diagnosis of FIP. Presence of antibodies in effusion is correlated with the presence of antibodies in blood; thus, antibody titers in effusions are not very helpful. One study investigating the diagnostic value of antibody detection in CSF reported a very good correlation to the presence of FIP when compared with histopathology; however, two studies investigating a large number of cats presented to veterinary teaching hospitals revealed no significant difference in antibody titers in CSF from cats with neurologic signs due to FIP compared with cats with other neurologic diseases confirmed by histopathology.
Feline Coronavirus Reverse Transcriptase PCR:
FCoV reverse transcriptase PCR in blood is used with increasing frequency as a diagnostic tool for FIP. However, so far, no PCR has been developed that can definitively diagnose FIP. PCR can be false-negative (eg, because the assay requires reverse transcription of viral RNA to DNA before amplification of DNA, and degradation of RNA can occur due to contamination with ubiquitous RNAases) or false-positive (eg, the assay does not distinguish between virulent and avirulent FCoV strains and will not discriminate FCoV from coronaviruses of other species). Furthermore, viremia appears to occur not only in cats with FIP but also in healthy carriers. FCoV RNA has been detected in the blood of cats with FIP but also in healthy cats that did not develop FIP for as long as 70 mo. Therefore, the results of PCR tests in general must be interpreted carefully, and PCR cannot be used as a tool to definitively diagnose FIP.
PCR has been used to detect FCoV in fecal samples, and it is sensitive and useful to document that a cat is shedding FCoV in feces. The strength of the PCR signal in feces correlates with the amount of virus present in the intestines. These results can be useful to detect cats that chronically shed high virus loads and that pose a high risk in multicat households.
Immunostaining of Feline Coronavirus Antigen:
Direct staining of FCoVs within macrophages by immunofluorescence in cytocentrifuged effusions or immunohistochemistry in tissue is considered the most specific test to confirm FIP. Immunostaining cannot differentiate between the “harmless” FCoV and FIP-causing FCoV, but finding infected macrophages in characteristic pyogranulomatous lesions or in inflammatory effusions is highly associated with FIP. In a recent study in which a large number of cats with confirmed FIP and controls with other confirmed diseases were investigated, positive immunofluorescence staining of intracellular FCoV antigen in macrophages of the effusion was 100% predictive of FIP. Unfortunately, the NPV of the test is not very high (57%), which can be explained by low numbers of macrophages on effusion smears resulting in negative staining. Immunohistochemistry can be used to detect the expression of FCoV antigen in tissue and is also 100% predictive of FIP if positive. However, invasive methods (eg, laparotomy or laparoscopy) are usually necessary to obtain appropriate tissue samples. Either histology itself is confirmative, or immunohistochemistry staining of FCoV antigen in tissue macrophages can be used to diagnose FIP.
Treatment, Control, and Prevention:
Because of the high prevalence of FCoV antibodies in healthy cats, widespread antibody testing of healthy cats is not an appropriate screening test for FIP in pet cats. Nor should cats in shelters be screened for antibodies before adoption. No treatment of healthy antibody-positive cats has been shown to prevent development of FIP.
Treatment of cats with FIP remains frustrating and is limited to the cases that respond favorably within the first few days. The prognosis for a cat with FIP is very poor. In a prospective study including 43 cats with confirmed FIP, the median survival after the definitive diagnosis was 9 days. Some cats, however, may live for several months. Factors that indicate a poor prognosis and a short survival time are low Karnofsky score (index for quality of life), low platelet count, low lymphocyte count, high bilirubin concentration, and a large amount of effusion. Seizures are an unfavorable prognostic sign; they are significantly more frequent in cats with marked extension of the inflammatory lesions to the forebrain. Cats that show no improvement within 3 days after treatment initiation are unlikely to show any benefit from therapy, and euthanasia should be considered. Longer survival or remission from clinical signs is rare.
Supportive treatment is aimed at suppressing the immune overreaction, usually using corticosteroids. However, there are no controlled studies that indicate whether corticosteroids have any beneficial effect. Cats treated with corticosteroids have shown anecdotal improvement for as long as several months. Immunosuppressive drugs such as prednisolone (2–4 mg/kg/day, PO) are commonly used. More potent cytotoxic drugs such as cyclophosphamide (4 mg/kg/day) have also been suggested. Cats with large effusions benefit from removal of the fluid; injection of dexamethasone into the abdominal or thoracic cavity may follow (1 mg/kg/day until no effusion is present).
Cats with FIP should receive supportive therapy, including fluids and nutritional support, and their quality of life should be monitored. Anecdotal reports suggest that ozagrel hydrochloride, a thromboxane synthetase inhibitor that inhibits platelet aggregation, and pentoxifylline, a drug that decreases vasculitis and inhibits several cytokines (such as interleukins and TNF-α), may be beneficial in some cats.
Immune modulators (eg, Propionibacterium acnes, acemannan, tylosin, promodulin, interferon-α) have been used to treat cats with FIP. However, controlled trials are lacking, and anecdotal reports often lack definitive diagnosis. It has been suggested that these agents may benefit infected animals by restoring compromised immune function. However, a nonspecific stimulation of the immune system would seem to be contraindicated in FIP, because clinical signs develop and progress as a result of an immune-mediated response.
A number of studies have investigated effectiveness of various antiviral treatments in cats with FIP, including interferons and ribavirin. To date, none have proved to be very successful. See table: Drugs That Have Been Suggested for Use in Feline Infectious Peritonitis Cases
Interferons have been used frequently in cats with FIP. Human interferon-α has a direct antiviral effect, and in vitro antiviral efficacy against an FIP-causing FCoV strain has been demonstrated. In a controlled study, cats with confirmed FIP treated with interferon-α at 106 IU/kg in combination with Propionibacterium acnes survived for ~3 wk. Feline interferon-ω is available in some European countries and Japan. FCoV replication is inhibited by feline interferon-ω in vitro, but there was no statistically significant difference in the mean survival time of cats enrolled in a randomized placebo-controlled double-blind treatment trial. Cats survived for 3–200 days, regardless of whether they received the drug or placebo.
Drugs That Have Been Suggested for Use in Feline Infectious Peritonitis Cases
Management of Exposed Cats:
When a cat in a household develops FIP, all in-contact cats will have already been exposed to the same FCoV. Under natural circumstances, it appears that the FIP-causing virus strain is not excreted in such cases, and FIP is not transmitted from cat to cat. However, under experimental conditions it has been possible to transmit FIP-causing virus from a cat with FIP to in-contact cats. Still, it appears to be relatively safe for a cat with FIP to remain in the same household with cats that have already been in contact to the FCoV strain. However, it is not recommended to allow contact between a cat with FIP and any new “naive” cat. Kittens, which are more susceptible to FIP than adults, should not be introduced to households with a recent history of FIP.
If a cat has been euthanized or has died due to FIP, the owner should wait 2 mo before obtaining another cat. FCoV can remain infectious for at least 7 wk in the environment, particularly where litter boxes are in use. Other cats currently in the household are most likely infected with and shedding FCoV. Cats are commonly presented to the veterinarian for evaluation after contact with a cat with FIP or a suspected or known virus excretor. The owner may want to know the prognosis for the exposed cat or whether it is shedding FCoV. Such cats will likely be antibody positive, because 95%–100% of cats exposed to FCoV become infected and develop antibodies ~2–3 wk after exposure. A few cats may be resistant to FCoV infection. Some cats in FCoV-endemic multicat households continuously remain antibody negative. The mechanism of action for this resistance is unknown.
Although exposed cats will most likely have antibodies, this is not necessarily associated with a poor prognosis. Most cats infected with FCoV will not develop FIP, and many cats in single- or two-cat households will eventually clear the infection and become antibody negative in a few months to years (usually ~6 mo). If titers are monitored, cats should be retested (using the same laboratory) every 6–12 mo until the antibody test is negative. Some cats will remain antibody positive for years. The value of serial antibody or PCR testing is mostly limited to protocols aimed at creating FCoV-free closed catteries.
Management of Multicat Households:
In most multicat households with unusually high cat numbers, FCoV is endemic and FIP is almost inevitable. Households of <5 cats may spontaneously and naturally become FCoV-free, but in households of >10 cats per group, this is almost impossible because the virus passes from one cat to another, maintaining the infection. In these FCoV-endemic environments, such as breeding catteries, shelters, foster homes, and other multicat homes, there is virtually nothing to prevent FIP.
Various tactics have been used to eliminate FCoV from an endemic cattery. Reducing the number of cats (especially of kittens <12 mo old) and keeping suspected FCoV-contaminated surfaces clean can minimize population loads of the virus. Antibody or fecal PCR testing and removal of positive cats should be performed to stop exposure and reinfection of recovered cats. Approximately ⅓ of antibody-positive cats excrete virus; thus, every antibody-positive cat should be considered infectious. After 3–6 mo, antibody titers can be retested. Alternatively, PCR testing of (several) fecal samples can be performed to detect chronic FCoV carriers; these cats should be removed. In large, multicat environments, 40%–60% of cats shed virus in their feces at any given time. Approximately 20% will shed virus persistently. If a cat remains persistently PCR-positive for >6 wk, it should be placed in a single-cat environment or with other chronic shedders.
Kittens of FCoV-shedding queens are often protected from infection by maternally derived antibodies until they are 5–6 wk old. An early weaning protocol for prevention of FCoV infection in kittens has been proposed and consists of isolation of queens 2 wk before parturition, strict quarantine of queen and kittens, and early weaning at 5 wk of age. Early removal of kittens from the queen and prevention of infection from other cats may succeed in keeping kittens free of infection. Kittens should be taken to a new home (with no FCoV-infected cats) at 5 wk of age. Although straightforward in concept, the protocol requires quarantine rooms and procedures to ensure that new virus does not enter. Special care must be taken during this period to socialize the kittens. The success of early weaning and isolation depends on effective quarantine and low numbers of cats (<5) in the household.
Another possible approach is to maximize heritable resistance to FIP in breeding catteries. Genetic predisposition plays a role in the disease but is not completely understood. Full-sibling littermates of kittens with FIP have a higher likelihood of developing FIP than other cats in the same environment. A cat that has two or more litters in which kittens develop FIP should not be bred again. Particular attention should be paid to pedigrees of toms in which FIP is overrepresented. Because line breeding often uses valuable tomcats extensively, eliminating such animals may have an effect on improving overall resistance.
In shelters, prevention of FIP is virtually impossible unless cats are strictly separated and handled only through sterile handling devices (comparable to isolation units). Isolation is often not effective because FCoV is easily transported on clothes, shoes, dust, and cats. There appears to be significant correlation between the number of handling events outside the cages and stress and the percentage of antibody-positive cats. Studies have shown dramatic increases in fecal shedding of FCoV in infected cats after entering an animal shelter. Half of the cats that were originally FCoV-negative were shedding FCoV within 1 wk of entering the shelter environment. Shelters should have written information sheets or contracts informing adopters about FCoV and FIP. Personnel should understand that FCoV is an unavoidable consequence of endemic FCoV in multicat environments. Good husbandry practices and facilities that can be cleaned easily may minimize virus spread.
Vaccination:
A vaccine developed with a temperature-sensitive mutant of the FCoV strain DF2-FIPV, which is reported to replicate in the cool lining of the upper respiratory tract but not at higher internal body temperature, is available in the USA, Canada, and Europe. This vaccine is administered intranasally and produces local immunity (IgA antibodies) at the site where FCoV first enters the body (the oropharynx), as well as cell-mediated immunity. Vaccination in an FCoV-endemic environment or in a household with known cases of FIP is not effective, probably because most cats are already seropositive for FCoV. The vaccine is labeled for use beginning at 16 wk of age, which may be too late to protect kittens against FCoV in endemic populations. Most cats develop systemic antibodies after vaccination, thus making the establishment and control of an FCoV-free household difficult. The American Association of Feline Practitioners lists the FIP vaccine as “not recommended.”
Zoonotic Risk:
- Feline Infectious Peritonitis
- Overview of Feline Infectious Peritonitis