Zoonotic Diseases
Global Zoonoses a lists zoonotic bacterial, viral, fungal, and parasitic diseases, grouped by category. Many proven zoonoses, including some diseases that are rare in people, organisms that are maintained primarily in people, some primate diseases, and diseases caused by fish and reptile toxins have been omitted. The table is intended to give a general clinical picture of each disease; current medical texts or review articles should be consulted for a more complete description. Clinical signs are listed; asymptomatic infections can also be assumed to occur in most cases. An indication of the mortality rate among healthy individuals has been provided for many infections. However, there is almost always a chance of death whenever lesions can become generalized, vital organs may be affected, secondary infections occur, and/or the patient is immunosuppressed. The mortality rate is often influenced by the availability of medical care, and it is generally lower when advanced medical support is available. The risk of death from some bacterial diseases with high mortality rates can be nearly eliminated with prompt antibiotic treatment.
If a disease is known to have unusual manifestations or to be particularly common and/or severe in immunocompromised people, this has been noted. In addition to these diseases, many pathogens can cause more severe disease and/or unusual signs in immunocompromised patients. Information on the geographic range of an organism should be taken as a rough guide. The precise ranges of many pathogens have not been completely determined. Organisms may also expand their range or be eradicated from areas where they were once abundant. In this table, “worldwide” indicates those organisms that are widespread and found on all major continents, although they may absent from some areas (eg, polar regions or some islands). In some cases, organisms indicated as being present on a continent may nevertheless have a limited distribution.
Global Zoonoses a
|
Disease |
Causative Organism |
Animals Involved |
Known Distribution |
Probable Means of Spread to People |
Clinical Manifestations in People |
|
|
|||||
|
|
|||||
|
Bacterial Diseases |
|||||
|
Actinomycosis (see Actinomycosis) |
Actinomyces bovis and other species in animals may affect people, but most human infections are caused by commensals of people, especially Actinomyces israelii |
Mammals |
Worldwide; very rare in people |
Probably contact; actinomycosis usually disseminates from endogenous human flora |
Granulomas, abscesses, skin lesions; chronic bronchopneumonia; abdominal mass that may mimic a tumor; endocarditis; sepsis |
|
Anthrax (see Anthrax) |
Bacillus anthracis |
Mainly in cattle, sheep, goats, horses, wild herbivorous animals; virtually all mammals and some birds are susceptible to high dose |
Worldwide but distribution is focal; common in Africa, Asia, South America, Middle East, parts of Europe |
Occupational contact exposure (abraded skin, mechanical transmission by biting flies, other routes); ingestion/foodborne, rarely airborne |
Early signs vary with route of inoculation; papule to ulcerative skin lesions; mild to severe gastroenteritis ± hematemesis, bloody diarrhea, ascites (abdominal GI form); sore throat, dysphagia, fever, neck swelling, mouth lesions (oropharyngeal GI form); pneumonia; all may progress to sepsis, meningitis; untreated cases fatal in 5%–20% (cutaneous) to 100% (inhalation) |
|
Arcobacter infections |
Arcobacter butzleri, A cryaerophilus, A skirrowii, possibly others |
Poultry, cattle, pigs, sheep, horses, shellfish; some studies detected these organisms in dogs and/or cats |
Worldwide |
Ingestion of contaminated water, undercooked meat (especially poultry) has been suggested |
Gastroenteritis; bacteremia, mainly in patients with chronic illnesses; endocarditis, peritonitis; emerging and incompletely understood |
|
Bordetellosis (see Respiratory Diseases of Pigs and Infectious Tracheobronchitis of Dogs) |
Bordetella bronchiseptica |
Dogs, rabbits, cats, pigs, guinea pigs, other mammals |
Worldwide; uncommon in people |
Exposure to saliva or sputum, aerosols |
Sinusitis, bronchitis, pertussis-like illness; pneumonia and disseminated disease (eg, endocarditis, peritonitis, meningitis), usually in immunocompromised; wound infection |
|
Borreliosis (see Lyme Borreliosis) |
|
|
|
|
|
|
—Lyme disease |
Borrelia burgdorferi sensu lato complex (B burgdorferi sensu stricto, B garinii, B afzelii, B spielmanii, B japonica) |
Wild rodents, insectivores, hedgehogs, hares, other mammals; birds are reservoirs for agent; deer are hosts for tick vector only (blood meals) |
Agents exist worldwide where Ixodes ticks are found; human cases have been reported in North America, Europe, Australia, parts of Asia, Amazon region of South America |
Ixodes spp bites |
Nonspecific febrile illness early; target skin lesions in many; may progress to arthritis, neurologic, cardiac, and/ or skin signs (acrodermatitis chronica atrophicans); syndromes may vary with infecting agent |
|
—Tickborne relapsing fever |
B recurrentis, B crocidurae, B turicatae, B hermsii, B persica, B hispanica, others; some species such as B duttoni are human pathogens and not zoonotic |
Wild rodents, insectivores, possibly birds |
Africa, Asia, Europe, Americas; species varies with region |
Tick bites (mainly Ornithodoros spp) |
High fever, malaise, headache, myalgia, chills; neurologic signs or abortion possible; recurring episodes, often milder, after a symptom-free period; death in 2%–5% |
|
—Southern tick-associated rash illness |
Etiology uncertain; various Borrelia spp suggested |
|
USA; most cases in southeast |
Tick bite (Amblyomma americanum) |
Resembles Lyme disease |
|
Brucellosis (see Brucellosis in Large Animals and see Brucellosis in Dogs) |
Brucella abortus |
Cattle, bison, water buffalo, African buffalo, elk, deer, sheep, goats, camels, South American camelids; other mammalian spillover hosts |
Once worldwide, now eradicated from domestic animals in some countries or regions; reservoirs in wildlife in some disease-free areas |
Ingestion (especially unpasteurized dairy products or undercooked meat), contact with mucous membranes and broken skin; strain 19 vaccine |
Extremely variable, subacute and undulant to sepsis; often nonspecific febrile illness with drenching sweats early; arthritis, spondylitis, epididymo-orchitis, endocarditis, neurologic, other syndromes if chronic; case fatality 5% in untreated |
|
|
B melitensis |
Goats, sheep, camels; other mammalian spillover hosts |
Asia, Africa, Middle East, Mexico, Central and South America, some parts of Europe |
Ingestion (including unpasteurized dairy products or undercooked meat), contact with mucous membranes and broken skin; Rev 1 vaccine |
As above; this species highly pathogenic for people |
|
|
B suis biovars 1–4; biovar 5 has not been reported in people |
Swine and wild pigs (biovars 1, 2, 3), European hares (biovar 2), reindeer and caribou (biovar 4); B suis also in some other mammals |
Biovars 1 and 3 worldwide in swine-raising regions except eradicated or nearly eradicated from domestic pigs in some countries; biovar 2 in wild boar in Europe; biovar 4 in Arctic |
Ingestion, direct contact with mucous membranes and broken skin |
As above |
|
|
B canis |
Dogs; evidence of infection in wild canids including coyotes, foxes |
Worldwide; rare in people |
Probably via ingestion or contact with mucous membranes, broken skin; close contact, especially with animals that recently aborted or gave birth |
Probably as above |
|
|
B pinnipedialis and B ceti |
Marine mammals |
Atlantic, Arctic, and Pacific oceans; Mediterranean sea |
Laboratory exposure; sources of other infections unknown (possibly contact with animals or exposure to seawater); rare or underdiagnosed in people |
Few cases known: mild to severe febrile illness, similar to that caused by other Brucella spp; neurobrucellosis with headache and chronic neurologic signs; spinal osteomyelitis |
|
Campylobacter enteritis (see Enteric Campylobacteriosis) |
C jejuni, C coli, occasionally other species; some strains of C jejuni seem to have broader host ranges than others |
Poultry, cattle, swine, dogs, cats, rodents, other mammals, wild birds |
Worldwide |
Foodborne (especially poultry and other meats, unpasteurized dairy products); waterborne; contact with infected animals (fecal/oral) |
Gastroenteritis from mild cases to fulminating or relapsing colitis; occasional sequelae such as reactive arthritis; occasionally, other syndromes, including sepsis |
|
Campylobacter fetus infection |
C fetus subsp fetus (most cases), C fetus subsp testudinum (proposed name); possibly C fetus subsp venerealis |
C fetus subsp fetus and C subsp venerealis in cattle, sheep, goats; C fetus subsp testudinum in reptiles |
Worldwide |
Probably direct contact or ingestion; often unknown |
Opportunist; sepsis, meningitis, endocarditis, abscesses, other systemic infections in elderly, immunocompromised, or infants; abortions, preterm births in pregnant women, neonatal sepsis; gastroenteritis not prominent in most cases |
|
Capnocytophaga infection |
C canimorsus, C cynodegmi |
Dogs, cats |
Probably worldwide |
Bites or scratches |
Fever, localized infections to bacteremia or sepsis, endocarditis, meningitis; often in immunocompromised or elderly |
|
Cat scratch disease |
Bartonella henselae; B clarridgeiae and other Bartonella species also implicated rarely in cat scratch disease or other conditions (eg, endocarditis) |
Cats and other felids; other Bartonella spp in canids, rodents, rabbits, other animals |
Worldwide |
Often associated with scratches, bites, especially from cats; potential for other exposures to broken skin via saliva; exposure of conjunctiva |
Lymphadenopathy (may be absent in elderly), fever, malaise, skin lesions at inoculation site in immunocompetent, usually self-limiting with complications (eg, endocarditis, neuroretinitis, neurologic disease) uncommon; inoculation into eye results in conjunctivitis ± ocular granuloma and local lymphadenopathy; risk of bacteremia, disseminated disease, bacillary angiomatosis in immunosuppressed |
|
Chlamydiosis (see also Psittacosis below) |
Chlamydia ( Chlamydophila) abortus, C felis |
C abortus in sheep, goats, cattle, other mammals; C felis in cats |
C felis worldwide; C abortus in most sheep-raising areas but not Australia or New Zealand |
Contact with animals; C abortus probably contact with pregnant or aborting ruminants |
C abortus: abortions, septicemia; C felis suspected agent of keratoconjunctivitis, also implicated in other conditions (controversial) |
|
Clostridial diseases (see Clostridial Diseases) |
Clostridium difficile; some ribotypes found in animals have been implicated as potential zoonoses |
Ribotypes from some calves, pigs, dogs are identical to some ribotypes found in people |
Worldwide |
Possible zoonosis; from contact or ingestion in contaminated meat; also from environment and contact with infected people |
Gastroenteritis, varying in severity from diarrhea to fulminant colitis, usually in conjunction with antibiotic use |
|
|
Clostridium perfringens, type A (most common), C, or D; environmental or endogenous source, with some potential for zoonotic transmission |
Domestic and wild animals, people |
Worldwide |
Foodborne (usually type A); nonfood-associated intestinal infection; wound contaminant, usually environmental; may be endogenous in debilitated from GI or urogenital tract |
Foodborne gastroenteritis, usually brief, self-limited except in debilitated; nonfood-related intestinal infection with prolonged diarrhea, sometimes bloody, mainly in elderly after antibiotics; life-threatening necrotic enteritis, often in debilitated; gas gangrene, sepsis; necrotic enteritis, gas gangrene, sepsis are fatal if not treated |
|
Corynebacterium ulcerans and C pseudotuberculosis infections |
C ulcerans, C pseudotuberculosis |
C ulcerans in cattle, pigs, small ruminants, dogs, cats, ferrets, other domestic and wild animals; C pseudotuberculosis in sheep, goats, cattle, horses, camelids, other mammals |
Probably worldwide; uncommon in people but may be increasing |
Direct contact, consumption of unpasteurized milk products |
Acute upper respiratory illness with sinusitis, sore throat, tonsillitis, or more severe pharyngitis resembling diphtheria (pseudomembranous pharyngitis); cardiorespiratory complications possible; peritonitis; isolated skin infection; some cases serious or fatal |
|
Dermatophilosis (see Dermatophilosis) |
Dermatophilus congolensis |
Cattle, horses, deer, sheep, goats, other mammals |
Worldwide, especially in warmer regions |
Usually direct contact with lesions; mechanical transmission on arthropod vectors, fomites possible |
Pustular desquamative dermatitis, other skin lesions |
|
Enterohemorrhagic Escherichia coli infectionsb |
E coli O157:H7; also implicated are types O157:H, and members of serogroups O26, O103, O104, O111, O145, and others |
Especially cattle, sheep; also goats, bison, deer, pigs, other species of mammals, birds |
Worldwide |
Ingestion of undercooked meat (especially ground beef), vegetables or water contaminated with feces; direct contact with feces or contaminated soil |
Diarrhea or hemorrhagic colitis; up to 15% of patients with hemorrhagic colitis progress to hemolytic uremic syndrome (HUS); case fatality rate for HUS is 3%–5%, higher in some populations (eg, 5%–10% in children, up to 50% in elderly) |
|
Erysipeloid (see Erysipelothrix rhusiopathiae Infection) |
Erysipelothrix rhusiopathiae |
Swine, sheep, cattle, rodents, marine mammals; many other domestic and wild mammals and marsupials, birds (including poultry), reptiles, fish, mollusks, crustaceans |
Worldwide |
Contact with animal products; via skin, usually after scratch or puncture wound; contaminated soil (survives for weeks to months) |
Localized cellulitis, usually self-limiting, often on hands; generalized skin lesions (uncommon); arthritis, often in finger joints near skin lesion; endocarditis (with high mortality, 38%); generalization with sepsis, other syndromes uncommon and often in immunocompromised |
|
Glanders (see Glanders) |
Burkholderia mallei |
Equids are reservoirs; felids, many other domesticated and wild mammals also susceptible |
Middle East, Asia, Africa and South America |
Contact with infected animals, tissues through broken skin, mucous membrane; ingestion; inhalation |
Mucous membrane or skin lesions; pneumonia and pulmonary abscess; sepsis; chronic abscesses, nodules, ulcers in many organs, weight loss, lymphadenopathy; case fatality rate varies with form, but >95% in untreated septicemia |
|
Helicobacter infection |
H pullorum, H suis, other species suspected as zoonoses |
Poultry (H pullorum), rodents (H pullorum and other species), pigs (H suis), dogs (H canis), many other mammals |
|
Uncertain; possibly ingestion of undercooked meat or direct contact |
Gastroenteritis or diarrhea, liver disease; bacteremia in immunosuppressed patients |
|
Leprosy (see Mycobacterial Infections Other than Tuberculosis) |
Mycobacterium leprae |
Armadillos; nonhuman primates (rare) |
Armadillos in parts of southern USA, Mexico, South America; nonhuman primates in Africa, possibly other locations; only human reservoirs in other areas |
Transmission of animal leprosy to people likely |
Various skin lesions, sensory nerve lesions and deficits, nasal mucosal lesions; mild, self-limiting to progressive destruction |
|
Leptospirosis (see Leptospirosis) |
Leptospira spp |
Domestic and wild animals; reservoir hosts include rodents, dogs, cattle, pigs, farmed red deer, others |
Worldwide |
Occupational and recreational exposure, or exposure to rodent-contaminated material in urban locations; especially skin, mucous membrane contact with contaminated urine, infected fetuses, or reproductive fluids; water- and foodborne |
Asymptomatic to severe, sometimes biphasic; nonspecific febrile illness followed by aseptic meningitis or icteric form (especially liver, kidney, CNS involvement, hemorrhages possible); pulmonary hemorrhage and edema, other syndromes; uveitis can be sequela; case fatality rate varies with syndrome (uncommon in aseptic meningitis, 5%–15% in icteric form, 30%–60% in severe pulmonary form) |
|
Listeriosis (see Listeriosis) |
Listeria monocytogenes (types most often associated with disease are ½a, ½b, 4b), Listeria ivanovii (rare) |
Numerous mammals, birds, fish, crustaceans |
Worldwide |
Foodborne, especially unpasteurized dairy products, raw meat and fish, vegetables, processed foods contaminated after processing; ingestion of contaminated water, soil; direct contact with infected animals; nosocomial in hospitals, institutions |
Acute, self-limited febrile gastroenteritis or mild, flu-like illness; ocular disease, conjunctivitis; abortion, premature or septicemic newborn if infected during pregnancy; meningitis, meningoencephalitis, septicemia in elderly, immunosuppressed, and infants; papular or pustular rash ± fever, chills in healthy adults after handling infected fetuses |
|
Melioidosis (Pseudoglanders, see Melioidosis) |
Burkholderia pseudomallei (other species of soil-associated Burkholderia, such as B oklahomensis sp nov in North America, rarely linked to human infections) |
Sheep, goats, swine; occasional cases in many other terrestrial and aquatic mammals; also reptiles, some birds including parrots, tropical fish |
Asia, Africa, Australia, South America, Middle East, Caribbean |
Wound infection, inhalation, and ingestion; organisms live in soil and surface water; most cases are acquired from environment, but direct transmission from animals is possible |
Mimics many other diseases; acute localized infections, including skin lesions, cellulitis, abscesses, corneal ulcers; pulmonary disease, septicemia, internal organ abscesses; often occurs in immunocompromised; case fatality rate varies with form, >90% in untreated septicemia |
|
Methicillin-resistant Staphylococcus aureus (MRSA) infections |
S aureus that carry mecA gene; some strains maintained in animals (eg, livestock-associated CC398), other strains mainly in people but animals can become carriers |
Pigs (major reservoirs for livestock-associated strain CC398, also carry ST9); cats, dogs mainly acquire strains from people; MRSA also reported in other mammals, including horses, cattle; birds, including poultry, psittacines; turtles |
Worldwide; can be reverse zoonosis or zoonosis; major strains in animals can vary with region |
Usually by direct contact (typically with asymptomatic carrier animals); other routes also described; can be nosocomial in hospitals |
Opportunist; localized skin and soft-tissue infections, invasive disease including septicemia, toxic shock syndrome; mortality varies with syndrome and success in finding antibiotic |
|
Mycobacteriosis (see Tuberculosis and other Mycobacterial Infections) |
Mycobacterium avium complex |
Many species of mammals, some birds |
Worldwide |
Environmental, mainly from water, and/or soil; infection common to people and animals |
Soft-tissue and bone infections; cervical lymphadenitis; pulmonary disease, often in immunocompromised or those with preexisting lung conditions; disseminated in immunocompromised, especially AIDS patients with uncontrolled disease |
|
|
M avium paratuberculosis |
Cattle, sheep, goats, camelids, deer, other ruminants; rabbits and other nonruminants; corvids |
Worldwide |
Ingestion; accidental injection of vaccine |
Postulated involvement in Crohn’s disease after ingestion (controversial); severe local reaction if vaccine accidentally injected |
|
|
Mycobacteria other than tuberculosis (includes M simiae, M kansasii, M xenopi, M scrofulaceum, M szulgai, M chelonae, M marinum, M ulcerans, others) |
Cattle, other ruminants; swine, cats, dogs, koalas, other mammals, amphibians, reptiles (uncommon), fish; predominant Mycobacterium spp vary with host |
Worldwide; distribution varies with the organism |
Environmental, from water and/or soil |
Same syndromes as M avium complex; some organisms tend to be associated with certain syndromes (eg, M marinum, M ulcerans, with ulcerative or nodular dermatitis) |
|
Mycoplasma infections |
Mycoplasma spp |
Livestock, nonhuman primates, marine mammals, cats, dogs, rodents, other mammals |
Worldwide; zoonotic infections rare |
Direct contact; bites; wound contamination, including accidental inoculation |
Asymptomatic carriage; cellulitis; other syndromes, including respiratory disease, septic arthritis, septicemia have been reported, especially in immunocompromised |
|
Pasteurellosis (see Pasteurellosis of Sheep and Goats and see Pasteurellosis) |
Pasteurella multocida and other species |
Many species of domestic and wild animals, including dogs, cats, livestock, rabbits, birds |
Worldwide |
Wounds, scratches, bites, close contact with mucus membranes |
Wound infections, cellulitis most common; other syndromes possible, including osteomyelitis, septic arthritis, sepsis, meningitis, respiratory disease; systemic conditions more common in immunocompromised |
|
Plague (see Plague) |
Yersinia pestis |
Rodents (eg, squirrels, prairie dogs, rats) and lagomorphs (pikas in Asia) are main reservoir; many mammals can be incidental hosts; cats and wild felids especially susceptible |
Foci in North and South America, Asia, Middle East, and Africa |
Flea bites, aerosols, handling infected animals or tissues (contact with broken skin or mucous membranes), bites or scratches, eating uncooked infected tissues |
Febrile flu-like syndrome with swollen, very painful draining lymph node(s) (buboes); pneumonia; sepsis can occur in either bubonic or pneumonic form; case fatality rate in untreated 40%–70% (bubonic) to 100% (pneumonic); < 5% mortality if bubonic form treated early |
|
Psittacosis and ornithosis (see Avian Chlamydiosis) |
Chlamydia (Chlamydophila) psittaci |
Psittacine birds (especially parakeets, cockatiels), pigeons, turkeys, ducks, geese, and other domestic or wild birds; mammalian strains of C psittaci also exist (zoonotic potential still undetermined) |
Worldwide |
Inhalation of respiratory secretions or dried guano |
Influenza-like febrile illness with nonproductive cough that may progress to pneumonia; complications, including endocarditis, myocarditis, meningoencephalitis, hepatitis, glomerulonephritis, and other organ dysfunction; sepsis; some cases fatal if untreated, <1% with treatment |
|
Rat bite fever |
Streptobacillus moniliformis |
Rodents; might also be transmitted by carnivores (eg, dogs, cats, ferrets), which are probably infected or transiently colonized from rodents |
Probably worldwide |
Bites and scratches; handling or kissing a rodent, exposure to rodent urine; can be waterborne or foodborne |
Fever, severe myalgia and joint pain, headache, rash, sometimes GI signs; complications, including polyarthritis (usually but not always sterile), hepatitis, endocarditis, focal abscesses, sepsis possible if untreated; overall case fatality rate 10%–13% if untreated |
|
|
Spirillum minus |
Rodents; might also be transmitted by carnivores, which are probably infected or transiently colonized from rodents |
Organism is common only in Asia |
Mainly bites and scratches |
As above, but indurated, often ulcerated lesion at inoculation site; can relapse; some (minority) may have distinctive rash (large violaceous or reddish macules); polyarthritis is rare; overall case fatality rate 7%–10% if untreated |
|
Salmonellosis (see Salmonellosis) |
Salmonella enterica and S bongori (> 2,500 serovars) |
Widespread in mammals, birds, reptiles, amphibians, including domestic species; also in crustaceans; higher-risk pets for human exposure may include reptiles, amphibians, young poultry, some exotic mammals |
Worldwide |
Foodborne infection or fecal-oral; some cases of occupational and recreational exposure |
Gastroenteritis to sepsis; focal infections possible; especially severe in the elderly, young children, or immunocompromised |
|
Streptococcal infections |
Streptococcus spp, including S suis, S equi zooepidemicus, S canis, S iniae, possibly others |
S suis in swine; S equi zooepidemicus in horses; S canis in dogs, cats; S iniae in fish; each species can also be found in other animals |
Worldwide |
Ingestion, especially of unpasteurized dairy products, pork; direct contact often through broken skin; the human pathogen S pyogenes can also colonize bovine udder and be transmitted in milk |
Skin and soft-tissue infections; pharyngitis; other conditions, including pneumonia, meningitis, arthritis, endocarditis, streptococcal toxic shock syndrome, sepsis |
|
Tuberculosis (see also mycobacteriosis, above, see Tuberculosis and other Mycobacterial Infections, and see Tuberculosis) |
Mycobacterium bovis |
Cattle, bison, African buffalo, cervids, brushtail opossums, badgers, kudu can be reservoirs; swine and many other mammals can be spillover hosts |
Once worldwide, now eradicated or rare in some countries |
Ingestion (unpasteurized dairy products, undercooked meat including bushmeat), inhalation, contamination of breaks in the skin |
Skin lesions, cervical lymphadenitis (scrofula), pulmonary disease; genitourinary disease; can affect bones and joints, meninges; gastroenteritis |
|
|
Mycobacterium caprae |
Mainly goats, also infects other ruminants; can occur in other mammals, including pigs, horses, cervids, camels, carnivores |
Reported mainly in Europe |
Thought to be ingestion or direct contact with livestock, similarly to M bovis |
Extrapulmonary conditions, including skin lesions, meningitis, lymphadenitis, pericarditis, urinary, dissemination; also pulmonary disease |
|
|
Mycobacterium microti |
Rodents thought to be reservoir; can occur in domestic animals, including cats, dogs, ferrets, livestock |
Appears to be rare human zoonosis |
|
Most reported cases have been pulmonary; can also cause extrapulmonary disease |
|
Tularemia (see Tularemia) |
Francisella tularensis subsp tularensis more virulent, F tularensis subsp holarctica (F tularensis type B) less virulent, F tularensis subsp mediasiatica, F tularensis subsp novicida |
Rabbits, rodents, cats, sheep, other mammals, birds, reptiles, fish; often in wild animals |
F tularensis subsp tularensis almost exclusively in North America; F tularensis subsp holarctica in North America, Europe, Asia; F tularensis subsp mediasiatica in Central Asia; F tularensis subsp novicida reported in North America, Australia, Spain |
Contact with mucous membranes, broken skin; insect bites (tabanids, mosquitoes, hard ticks); fomites; ingestion in food or water; inhalation |
Nonspecific febrile illness, lymphadenitis; ulcerative skin lesions, exudative pharyngitis and stomatitis, conjunctivitis, gastroenteritis, respiratory signs or pneumonia, sepsis; case fatality rate 5% (localized disease, untreated) to >50% (untreated typhoidal form or severe respiratory disease) |
|
Vibriosis |
Vibrio parahaemolyticus |
Marine and estuarine shellfish, fish; also environmental in aquatic environments |
Worldwide |
Ingestion; wound infections |
Gastroenteritis; dysentery (especially in some geographic regions); wound infections (mild to severe, including necrotizing fasciitis); sepsis; severe wound infections and sepsis usually in immunocompromised or those with liver disease (case fatality rate for sepsis 29%) |
|
|
V vulnificus |
Marine shellfish, crustaceans (eg, shrimp), fish; also environmental in aquatic environments |
Worldwide; human cases have been reported in North America, Europe, Asia |
Ingestion (often raw oysters); wound infection from water or handling hosts |
Wound infections from mild, self-limited lesions, bullae to cellulitis, myositis; necrotizing fasciitis; gastroenteritis; sepsis, usually in immunocompromised or those with liver disease, other debilitating illnesses; case fatality rate for sepsis >50%, and up to 25% for wound infections |
|
Vibriosis (continued) |
V cholerae O1/O139 (epidemic strains) |
Oysters, crabs, shrimp, mussels; most cases acquired from people |
Rare/absent to epidemic in different regions; one focus along USA Gulf Coast in shellfish |
Ingestion |
Mild to severe, voluminous diarrhea, vomiting, dehydration; severe cases fatal if untreated, but low mortality if treated |
|
|
V cholerae non-O1/O139 (nonepidemic strains) |
Oysters, other seafood; also environmental in aquatic environments |
Worldwide |
Ingestion; wound infection |
Gastroenteritis, usually mild and self-limited; wound infections; septicemia, usually in immunosuppressed or those with liver disease (case fatality rate for sepsis 47%–60% or higher) |
|
Yersiniosis |
Yersinia pseudotuberculosis |
Many species of mammals, including swine, dogs, cats, rodents, wild mammals, birds |
Agent probably worldwide; prevalence may vary between regions |
Ingestion of contaminated water, food (including meat [especially pork], vegetables); fecal-oral (animal contact); dog bite (rare) |
Gastroenteritis (enterocolitis); pseudoappendicitis (with mesenteric lymphadenitis, terminal ileitis, fever, abdominal pain); severe GI bleeding possible in some cases of colitis; pharyngitis; sequelae may include erythema nodosum, reactive arthritis; sepsis, especially in elderly or immunocompromised |
|
|
Y enterocolitica; not all serotypes are pathogenic |
Many domestic and wild mammals, including rodents; some birds, reptiles, amphibians; zoonotic serotypes most common in pigs (major zoonotic source), pathogenic types also occur in dogs, cats |
Worldwide; prevalence of human disease may vary between regions (commonly reported in Europe) |
Ingestion |
Gastroenteritis with watery diarrhea especially in young children, bloody feces uncommon; pseudoappendicitis; sequelae may include erythema nodosum, reactive arthritis; sepsis, other syndromes |
|
|
|||||
|
|
|||||
|
Rickettsial Diseases |
|||||
|
Human ewingii ehrlichiosis (formerly granulocytic ehrlichiosis) |
Ehrlichia ewingii |
Dogs, deer proposed |
Southeastern and south central USA; has been detected in South America |
Ticks, including Amblyomma americanum |
Few cases described; fever, headache, malaise, myalgia, nausea, vomiting; many patients were immunosuppressed |
|
Human monocytic ehrlichiosis (see Ehrlichiosis and Related Infections) |
Ehrlichia chaffeensis |
Deer are probably major reservoir in North America, dogs and other canids, lemurs, other mammals can also be infected |
North America; also reported in South America, Asia, and Africa |
Ticks, including Amblyomma americanum |
Asymptomatic to nonspecific febrile illness; rash in many pediatric cases, some adults; may progress to prolonged fever, renal failure, respiratory distress, hemorrhages, cardiomyopathy, neurologic signs, multiorgan failure; more severe in immunosuppressed, elderly; estimated case fatality rate 2%–3% |
|
Human granulocytic anaplasmosis (formerly human granulocytic ehrlichiosis) |
Anaplasma phagocytophilum (formerly Ehrlichia phagocytophilum and E equi) |
Wild rodents, deer may be reservoirs in North America; livestock, wild ungulates, wild rodents may be reservoirs in Europe; many other animals (eg, equids, ruminants, dogs, cats, birds) can also be infected |
Worldwide |
Tick bites (Ixodes spp) |
Resembles human monocytic ehrlichiosis; often asymptomatic to mild in immunocompetent; rash uncommon; estimated case fatality rate <1% |
|
Infection by other Ehrlichia species |
E canis, E muris–like organism implicated rarely in human illness |
Dogs and other canids thought to be reservoirs for E canis, might also occur in felids; rodents may be reservoirs for E muris |
E canis worldwide; E muris Eastern Europe to Asia; E muris–like organism in North America |
Ticks (E canis transmitted by Rhipicephalus sanguineus, E muris by Haemaphysalis flava and Ixodes persulcatus complex) |
Rare cases of febrile illness, in both healthy and immunosuppressed |
|
Q fever (Query fever, see Coxiellosis) |
Coxiella burnetii |
Sheep, cattle, goats, cats, dogs, rodents, other mammals, birds, ticks |
Worldwide |
Mainly airborne; exposure to placenta, birth tissues, animal excreta; occasionally ingestion (including unpasteurized milk); tickborne infections probably rare or nonexistent in people |
Febrile influenza-like illness; atypical pneumonia, hepatitis, endocarditis in some; possible pregnancy complications; overall case fatality rate 1%–2% if untreated |
|
Sennetsu fever |
Neorickettsia sennetsu |
Uncertain, possibly fish |
Japan, Malaysia, Laos, possibly other Asian countries |
Thought to be ingestion of raw fish |
Relatively mild, nonspecific, febrile illness, resembles infectious mononucleosis |
|
Spotted fever group of Rickettsia |
|
|
|
|
|
|
—African tick bite fever |
R africae |
Ungulates |
Sub-Saharan Africa, eastern Caribbean |
Bite of infected tick (mainly Amblyomma hebraeum, A variegatum, also A lepidum, possibly Rhipicephalus decoloratus,Rhipicephalus appendiculatus) |
Nonspecific febrile illness; painful regional lymphadenopathy in many; eschars often multiple; nuchal myalgia; sometimes sparse maculopapular and/or vesicular rash; deaths do not seem to occur |
|
—Mediterranean spotted fever; Boutonneuse fever; Tick bite fever; |
R conorii subsp conorii |
Dogs, rabbits implicated as reservoirs; other animals can be infected |
Europe, especially Mediterranean; cases reported in sub-Saharan Africa |
Bite of infected ticks (mainly Rhipicephalus sanguineus, also others), crushing tick |
Nonspecific febrile illness; eschar (typically single) may or may not be present; rash, often maculopapular, in most; life-threatening disseminated disease or neurologic signs possible but uncommon; case fatality rate 1%–3% if untreated |
|
—Israeli spotted fever, Astrakhan spotted fever, Indian tick typhus |
R conorii subsp israelensis (Israeli spotted fever), R conorii subsp caspia (Astrakhan spotted fever), R conorii subsp indica (Indian tick typhus) |
Reservoir hosts uncertain |
Israeli spotted fever in Middle East, reported in Europe; Astrakhan spotted fever in Russia, Kazakhstan; Indian tick typhus in Asia (Indian subcontinent) |
Bite of infected ticks (mainly Rhipicephalus spp), crushing tick |
Astrakhan spotted fever and Indian tick typhus resemble Mediterranean spotted fever, but Israeli spotted fever may be more severe |
|
—Fleaborne spotted fever; Cat flea typhus |
R felis (synonym ELB agent) |
Unknown; dogs have been suggested as possible amplifying hosts |
North and South America, Europe, Asia, Africa, probably worldwide |
Flea bites; mainly associated with Ctenocephalides felis (cat flea), also infects C canis and other fleas |
Few clinical cases have been described but resembles other spotted fevers; febrile illness; rash in most; eschar may be uncommon; most cases seem to be mild but CNS involvement, pneumonia possible |
|
—Queensland tick typhus |
R australis |
Bandicoots, rodents |
Australia |
Bite of infected Ixodes tick, especially I holocyclus, I tasmani |
Febrile illness, eschar may be present, rash (either maculopapular or vesicular) in most; mild in most, but serious disseminated disease, complications, death possible |
|
—Rickettsial pox |
R akari |
Mice; also rats, Korean voles |
Organism may be cosmopolitan; human cases seem to be uncommon |
Bite of infected rodent mites, Liponyssoides sanguineus |
Eschar (single) in most; febrile illness; maculopapular rash progresses to vesicular, pustular, resembles chickenpox; self-limiting |
|
—Rickettsia parkeri rickettsiosis |
R parkeri |
|
North America, detected in parts of South America |
Bite of infected ticks, Amblyomma maculatum; also found in other Amblyomma spp |
Resembles Rocky Mountain spotted fever (RMSF) but seems to be milder in most cases; differs from RMSF in that eschars occur in most cases (may be multiple), petechial rash does not seem to be characteristic |
|
—Rocky Mountain spotted fever (see Rocky Mountain Spotted Fever) |
R rickettsii |
Rodents, rabbits, opossums, and other small mammals might amplify; dogs can be infected |
Western hemisphere |
Bite of infected ticks, especially Dermacentor variabilis, D andersoni (D variabilis in USA); Amblyomma cajennense, A aureolatum in South America; Rhipicephalus sanguineus in Arizona, Mexico, and South America; also from crushing tick |
Moderate to severe febrile illness; macular to generalized petechial rash; edema in some; usually no eschar; neurologic, pulmonary, hemorrhagic, and kidney signs in some; sepsis; gangrene; case fatality rate 15%–30% or higher (up to 85%) if untreated, ~3% or less with treatment in North America but higher in parts of Brazil |
|
—Tickborne lymphadenopathy; Dermacentor necrosis-erythema-lymphadenopathy |
R slovaca, R raoultii |
Uncertain; wild boar may be involved |
Europe to Central Asia |
Bites of infected ticks; R slovaca especially in Dermacentor marginatus, D reticulatus; R raoultii in Rhipicephalus pumilio, D nuttalli, other Dermacentor spp |
Eschar, local lymphadenopathy; localized alopecia at bite site; mild illness, fever and rash uncommon; no deaths reported |
|
—Other tickborne species in spotted fever group |
R sibirica, R japonica, R helvetica, R honei, R heilongjiangensis, R aeschlimannii, R massiliae, R monacensi, R amblyommii, others |
Various vertebrates |
Worldwide; distribution varies by species |
Bites of ixodid ticks; specific vector varies by species |
Inoculation site eschar (most); febrile illness with headache, myalgia, sometimes other signs; rash; local lymphadenopathy (some species); major signs, risk of complications, severity vary with species of Rickettsia |
|
Typhus group of Rickettsia |
|
|
|
|
|
|
—Murine typhus; Fleaborne typhus |
R typhi (formerly R mooseri) |
Rats are major reservoir; cats, opossums, possibly dogs, other species in peridomestic cycle |
Worldwide, especially warmer regions |
Infected rodent fleas, usually via flea feces; cat fleas seem to be involved in some cycles |
Fever, severe headache, central rash (not always observed); other signs, including arthralgia, cough, nausea/vomiting in some; mortality rate 4% without treatment |
|
—Scrub typhus; Chigger-borne rickettsiosis |
Orientia tsutsugamushi and related species |
Rodents, insectivores |
Asia, Australia, islands of southwestern Pacific Ocean; cases are usually concentrated regionally in “typhus islands” |
Bite of infected larval trombiculid mites (chiggers) |
Eschar in some; rash, headache, fever, painful lymphadenopathy, body aches, interstitial pneumonitis, GI signs; pneumonia, neurologic signs or cardiac complications in some; mild to severe; convalescence prolonged; case fatality rate up to 30%–50% if untreated |
|
—Typhus |
R prowazekii |
Flying squirrels |
Eastern USA |
Squirrel lice or fleas suspected |
Nonspecific febrile illness, rash; GI signs in some; sepsis possible; appears to be somewhat milder than non-zoonotic typhus, which has a mortality rate of 20%–60% if untreated |
|
|
|||||
|
|
|||||
|
Fungal Diseases |
|||||
|
Aspergillosis; Allergic bronchopulmonary aspergillosis (see Aspergillosis) |
Aspergillus spp |
Birds and mammals |
Worldwide |
Environmental exposure (decaying vegetation or grains); infection common to people and animals, insignificant as zoonosis |
Allergic respiratory signs, especially in people with certain respiratory conditions or immunodeficiencies; allergic sinusitis; pneumonia sometimes with dissemination in immunocompromised (can be fatal); chronic pulmonary disease ± aspergilloma (fungus ball); localized infections of other organs, tissues |
|
Blastomycosis (see Blastomycosis) |
Blastomyces dermatitidis |
Dogs, cats, horses, marine mammals, other mammals |
Distribution in environment uncertain; clinical cases focal; locally acquired cases reported in parts of North America, Africa, Middle East, India |
Environmental exposure, organism is most common in moist soil; infection common in people and animals; also reported rarely by animal exposure |
Acute to chronic pulmonary disease; skin or bone lesions; meningitis, other syndromes, disseminated disease possible; course mild to severe, some cases fatal |
|
Coccidioidomycosis (see Coccidioidomycosis) |
Coccidioides immitis, C posadasii |
Cattle, sheep, horses, llamas, dogs, many other mammals |
Especially southwestern USA, Mexico, Central and South America; in arid or semiarid foci; some cases might be acquired outside usual foci |
Principally environmental exposure (inhalation of arthrospores), including fungal cultures; infection common in people and animals, one unusual case reported after necropsy of horse with disseminated disease |
Self-limited, febrile, flu-like illness, sometimes with cough, chest pain in healthy host; serious, possibly life-threatening pulmonary disease or disseminated infection with cutaneous/subcutaneous lesions, persistent meningitis or osteomyelitis, especially in immunocompromised |
|
Cryptococcosis (see Cryptococcosis) |
Cryptococcus neoformans var grubii, C neoformans var neoformans, C gattii |
Birds including pigeons, psittacines (mainly grows in guano; temporary colonization of intestinal tract also possible); clinical cases in cats, other mammals |
Worldwide |
Principally environmental exposure, especially pigeon nests for C neoformans, trees for C gattii; via inhalation or through the skin; infection common in people and animals, insignificant as zoonosis |
Respiratory signs, mild to severe, often self-limiting in healthy host but more likely to be severe in immunocompromised; dissemination with CNS disease, ocular signs, other syndromes, most often in immunocompromised; skin lesions, either localized from inoculation (uncommon) or from disseminated disease |
|
Histoplasmosis (see Histoplasmosis) |
Histoplasma capsulatum var capsulatum |
Dogs, cats, bats, cattle, sheep, horses, many other domestic and wild mammals, birds |
Worldwide; clinical cases often cluster in regional foci |
Principally environmental exposure, avian or bat feces encourage growth of organism; infection common in people and animals; insignificant as zoonosis |
Flu-like, febrile illness, usually self-limiting in healthy hosts; skin lesions; chronic pulmonary disease, usually with preexisting lung disease; dissemination in very young, elderly, immunocompromised |
|
|
H capsulatum var duboisii |
As above |
Africa |
As above |
Usually skin and subcutaneous lesions, osteolytic bone lesions but can disseminate |
|
Malassezia infection |
Malassezia spp |
Dogs, cats, other animals |
Worldwide |
Exposure to symptomatic animals; normal levels on skin not thought to be a significant risk |
Dermatitis; zoonotic strains might be implicated in fungemia in preterm neonates, other immunocompromised |
|
Ringworm (Dermatophytosis, see Dermatophytosis) |
Microsporum and Trichophyton spp |
Dogs, cats, hedgehogs, cattle, sheep, goats, horses, rodents, other mammals, birds, very rarely reptiles |
Worldwide |
Direct skin/hair contact with infected animals, fomites |
Skin and hair lesions, usually pruritic; rare skin dissemination in immunocompromised |
|
Sporotrichosis (see Sporotrichosis) |
Sporothrix schenckii |
Cats, other mammals, birds |
Worldwide; epizootics in cats in South America |
Primarily environmental in vegetation, wood, soil; inoculation from environment in penetrating wounds (splinters, bites, pecks), skin contact with lesions, especially in cats; bites, scratches, other close contact implicated during feline epidemics; inhalation rare |
Papules, pustules, nodules, ulcerative skin lesions, may follow course of draining lymphatics; mucosa can be affected; extracutaneous involvement, especially bones, joints; disseminated disease (including meningitis) can be seen in immunocompromised; acute or chronic pulmonary disease resembling tuberculosis after inhalation, especially with underlying lung disease (rare) |
|
|
|||||
|
|
|||||
|
Parasitic Diseases—Protozoans |
|||||
|
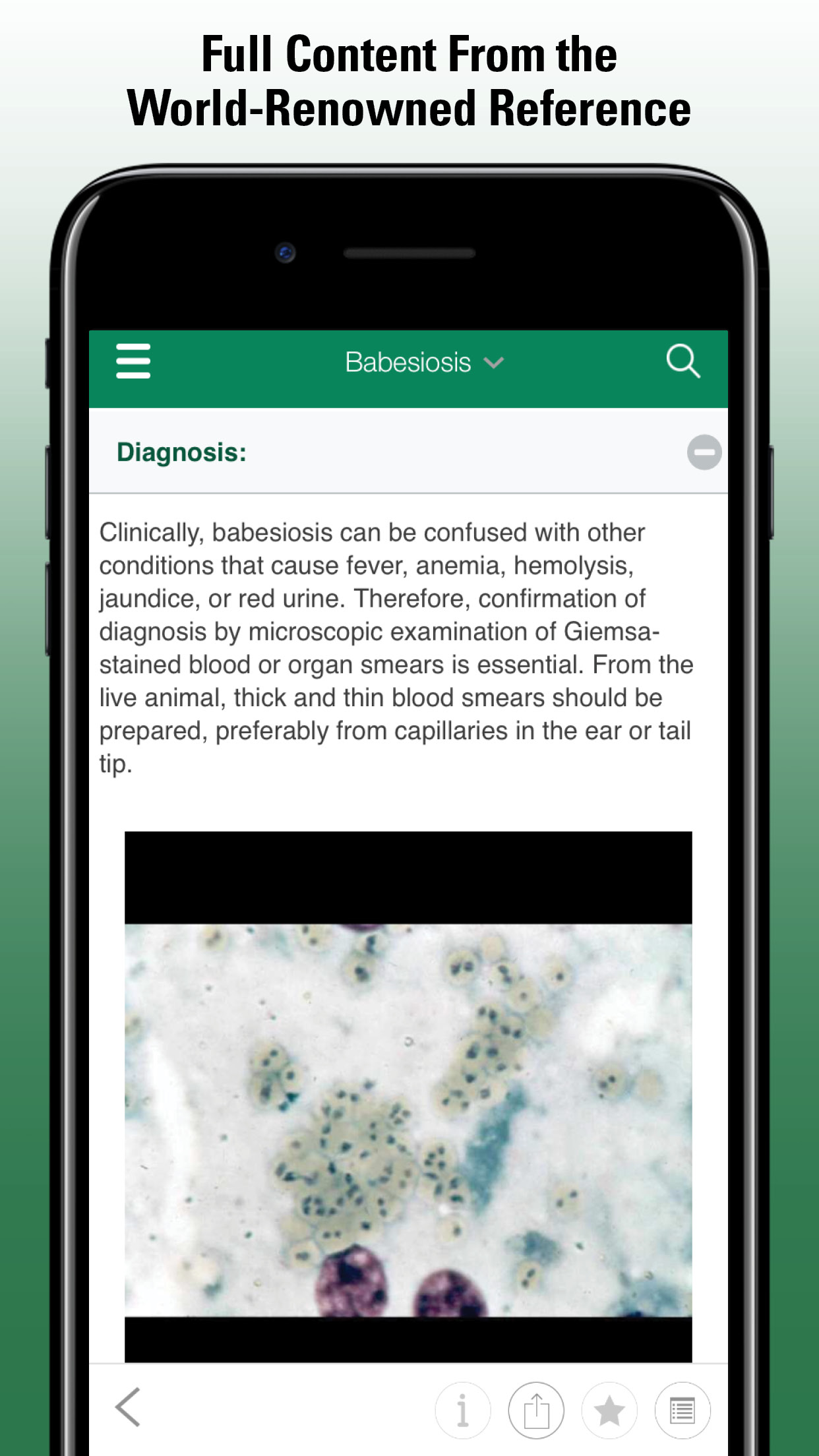
Babesiosis (see Babesiosis) |
Babesia microti complex, B duncani (formerly WA-1), and other species |
Rodents, insectivores, lagomorphs, some other mammals; reservoirs uncertain for some species |
Babesia spp worldwide in wild animals, many agents not identified to species; human illness due to B microti complex reported in North America (most), Europe, Asia, Australia |
Bite of infected Ixodes ticks for B microti |
Many immunocompetent patients may have mild to moderate flu-like, febrile illness; mild to severe hemolytic anemia, especially severe in immunocompromised and elderly; respiratory, hepatic, renal, and other organ dysfunction; recurrent or chronic infection may develop; dual infection with B burgdorferi may worsen both diseases; death possible in severe cases |
|
|
B divergens |
Cattle; B divergens or closely related organism in farmed reindeer, wild cervids |
Europe, possibly North Africa; similar organisms might be present in North America; reported in Asia (China) |
Tick bites (Ixodes ricinus) |
Usually in splenectomized; acute, severe hemolysis; persistent high fever, headache, myalgia, abdominal pain, sometimes GI signs; shock and renal failure; cases progress rapidly; usually fatal if untreated; milder flu-like cases have been reported in immunocompetent patients |
|
|
B bovis; uncertain zoonosis; some historical cases were probably B divergens |
Cattle, water buffalo, African buffalo, possibly other species |
Africa, Asia, Central and South America, Mexico, Australia, parts of Europe |
Tick bites (Rhipicephalus microplus and R annulatus) |
|
|
Balantidiasis |
Balantidium coli and related species |
Swine, rats, nonhuman primates, other animals |
Worldwide |
Ingestion, especially of water contaminated with feces |
Asymptomatic to mucoid, bloody feces; intestinal hemorrhage and perforation possible; rare extraintestinal cases |
|
Chagas’ disease (American trypanosomiasis, see Chagas’ Disease) |
Trypanosoma cruzi |
Opossums, lagomorphs, rodents, armadillos, dogs, cats, other wild and domestic mammals |
Western hemisphere—southern USA, Mexico, Central and South America |
Fecal material of reduviid bug in family Triatomidae contaminates bite wounds, abrasions, or mucous membranes; ingestion in contaminated food |
Acute disease—erratic fever, adenopathy, headache, myalgia, hepatosplenomegaly, swelling at inoculation site and eyelid; myocarditis or encephalitis in some; worse in immunocompromised Chronic form (in 10%–30% of patients)—cardiomyopathy, megaesophagus, megacolon, other forms; reported annual mortality rate in chronic form 0.2%–19% (higher rates from studies that include only cardiac patients) |
|
Cryptosporidiosis (see Cryptosporidiosis) |
Cryptosporidium parvum, C canis, C felis, C meleagridis, C cuniculus, C viatorum, C muris, and other species (C hominis and likely some genotypes of C parvum are adapted mainly to people) |
Cattle and other ruminants, dogs, cats, rabbits, other domestic and wild mammals, birds, reptiles, fish |
Worldwide |
Fecal-oral; ingestion of contaminated food and water; inhalation |
Self-limiting gastroenteritis in healthy; can be cholera-like and persistent in immunocompromised, with weight loss, wasting; cholecystitis; respiratory signs, pancreatitis, other syndromes mainly in immunosuppressed |
|
Giardiasis (see Giardiasis (Giardia)) |
Giardia intestinalis, also known as G duodenalis (formerly G lamblia); only some genotypes seem to have zoonotic potential |
Many domestic and wild mammals, including dogs, cats, ruminants, aquatic mammals such as beavers |
Worldwide |
Ingestion of water and less often food; direct fecal-oral (hands or fomites) |
Gastroenteritis, may be persistent |
|
Leishmaniosis —Visceral (Kalaazar; see Leishmaniosis) |
Leishmania infantum |
Wild canids and dogs are primary reservoirs, also in other mammals |
Asia, South America, Caribbean, Africa, the Middle East, Europe (Mediterranean spreading north), North America |
Bite of sand flies Phlebotomus and Lutzomyia spp |
Undulating fever, hepatosplenomegaly; some have cough, diarrhea, lymphadenopathy, weight loss, petechiae or hemorrhages on mucous membranes, nodular lesions or darkening of skin; pancytopenia; mild cases with only a few signs may resolve on their own, but most other cases fatal if untreated |
|
—Cutaneous and mucocutaneous |
L tropica complex (except L tropica, which is maintained in people), L braziliensis complex, L mexicana complex, others |
Dogs (L peruviana), rodents, various wild mammals act as reservoir hosts; other mammals can be infected |
Mediterranean, Asia, Africa, Middle East, Mexico to South America, Caribbean; localized focus in USA (Texas and Oklahoma) |
As above |
Papules to ulcers or nodules on skin ± mucous membranes; single or multiple lesions; localized or disseminated; may persist or recur; atypical forms in immunosuppressed; cutaneous form rarely fatal, mucocutaneous form can be disfiguring and may be fatal if pharynx affected |
|
Malaria of nonhuman primates |
Nonhuman primate–associated Plasmodium spp, P knowlesi, rarely P cynomolgi, others also potential zoonoses |
Old and New World monkeys, apes |
P knowlesi in Asia; other species exist in Central and South America, Asia, Africa |
Bite of anopheline mosquitoes |
Febrile episodes with chills; headache, myalgia, malaise, cough, nausea, vomiting, and other symptoms in some; cases range from mild, self-limiting to fatal (3% case fatality rate for P knowlesi) |
|
Microsporidiosis |
Microsporidia of Enterocytozoon bieneusi, Encephalitozoon cuniculi, E intestinalis, E hellem, others; both zoonotic and anthropnotic transmission reported for some agents |
Widespread in vertebrates, including primates, rabbits, rodents, dogs, cats, cattle, pigs, goats, birds, fish; also in invertebrates |
Worldwide |
Fecal-oral; direct contact; ingestion of contaminated food or water; aerosols; possibly vector-transmitted |
Keratitis; acute diarrhea (traveler’s diarrhea); chronic diarrhea in immunocompromised; may disseminate to systemic disease with variable symptoms in immunocompromised |
|
Rhinosporidiosis (see Rhinosporidiosis) |
Rhinosporidium seeberi; some strains may be host specific |
Natural hosts thought to be fish and amphibians; also found in various mammals, including horses, cattle, mules, dogs, and cats; birds |
Worldwide, especially in tropics; endemic in South America, Asia, and Africa |
Environmental exposure, probably water |
Nasal and other mucous membrane masses and polyps (mainly nose, nasopharynx, eye); may cause obstruction; rare disseminated disease with osteolytic lesions or affecting viscera; rare skin and subcutaneous lesions |
|
Sarcocystosis (Sarcosporidiosis, see Sarcocystosis) |
Sarcocystis suihominis, also called S meischeriana |
People, nonhuman primates are definitive hosts; swine are intermediate host |
Worldwide |
Ingestion of raw pork |
Gastroenteritis, usually mild, or asymptomatic |
|
|
S hominis, also called S fusiformis |
People, nonhuman primates are definitive hosts; cattle are intermediate host |
Worldwide |
Ingestion of raw beef |
Gastroenteritis, usually mild or asymptomatic |
|
|
Sarcocystis spp; S nesbitti may be one cause |
People are intermediate host; species of Sarcocystis and definitive host(s) are often unknown; definitive host for S nesbitti thought to be snakes |
Worldwide; symptomatic cases mainly in Asia, probably because of distribution of definitive host |
Assumed to be ingestion of oocysts shed in feces of definitive host(s) or sporocysts |
Main syndrome is myositis, acute and self-limited to chronic, moderately severe; also cough, arthralgia, transient pruritic rashes, headache, malaise, lymphadenopathy in some |
|
Toxoplasmosis (see Toxoplasmosis) |
Toxoplasma gondii |
Felidae, including domestic cats, are definitive hosts; essentially all other mammals (including livestock) and birds thought to be susceptible as intermediate hosts |
Worldwide |
Ingestion of oocysts shed in feces of infected cats (including contaminated soil, food, water) or ingestion of tissue cysts in undercooked meat or unpasteurized milk |
Lymphadenopathy or mild, febrile, flu-like syndrome or uveitis in immunocompetent, nonpregnant host; often severe in immunocompromised, with neurologic disease, chorioretinitis, myocarditis, pneumonitis, or disseminated disease; infection of fetus may result in CNS damage or generalized infection; abortions and stillbirths |
|
Trypanosomiasis (African sleeping sickness, see Trypanosomiasis) |
Trypanosoma brucei; T brucei rhodesiense is zoonotic; T brucei gambiense is primarily a human pathogen, although some animals (eg, pigs) can be infected and might serve as minor reservoirs |
T brucei rhodesiense reservoirs may include cattle, sheep, antelope, hyenas, lions, other wildlife, people; also isolated from other mammals |
Africa; common below the Sahara desert |
Bite of infected tsetse fly (Glossina spp) |
Painful chancre at bite site in some patients; intermittent fever, headache, adenopathy, rash, arthralgia; neurologic signs such as somnolence, seizures; cardiac complications possible; gambiense disease may last years; rhodesiense disease acute, may last weeks to months; both usually fatal without treatment |
|
|
|||||
|
|
|||||
|
Parasitic Diseases—Trematodes (Flukes) |
|||||
|
Clonorchiasis |
Clonorchis sinensis (Chinese liver fluke) |
Dogs, cats, swine, rats, other mammals are definitive hosts; fish (and snails) are intermediate hosts |
Asia |
Ingestion of undercooked infected freshwater fish or shrimp containing encysted larvae |
Cholecystitis symptoms, indigestion, diarrhea, mild fever; chronic infections associated with cirrhosis, pancreatitis, or cholangiocarcinoma |
|
Dicrocoeliasis |
Dicrocoelium dendriticum, possibly D hospes (lancet flukes) |
Ruminants, especially sheep, goats, cattle, occasionally other domestic and wild mammals are definitive hosts; land snails (first) and ants (second) are intermediate hosts |
D dendriticum on all major continents (may be focal); D hospes in Africa south of Sahara desert |
Ingestion of infected ants |
Abdominal discomfort, flatulent indigestion; occasionally GI signs (diarrhea, constipation, vomiting, pain); weight loss, fatigue; biliary obstruction, cholangitis, hepatomegaly, or acute urticaria possible |
|
Echinostomiasis |
Echinostoma revolutum, E ilocanum, E hortense, and other Echinostoma spp; Echinochasmus japonicus and other members of Echinostomatidae can also be zoonotic |
Cats, dogs, rodents, pigs, other mammals; birds, including poultry, are definitive hosts; fish, shellfish, tadpoles, snails are intermediate hosts |
Most human cases in Asia, Western Pacific; this group of parasites is widely distributed, including Europe, Americas, Middle East |
Ingestion of undercooked fish, shellfish, snails, or amphibians (frogs) |
Abdominal discomfort; diarrhea, especially in heavy infestation; malnutrition, anemia, edema may occur, especially in children; intestinal perforation has been reported |
|
Fascioliasis |
Fasciola hepatica |
Cattle, sheep, water buffalo, horses, rabbits, other herbivores are definitive hosts; snails are intermediate hosts |
Worldwide or nearly worldwide; previously thought to be mainly in temperate areas but may be more widely distributed |
Ingestion of contaminated greens, eg, watercress, or water that contains metacercariae |
Gastroenteritis, hepatomegaly, fever, urticaria possible acutely; biliary colic and obstructive jaundice in chronic cases; aberrant migration with extrahepatic signs (eg, pulmonary infiltrates, neurologic signs, lymphadenopathy, skin lesions or subcutaneous swelling) in some |
|
|
F gigantica |
Cattle, buffalo, goats, sheep, zebras, other mammals are definitive hosts; snails are intermediate hosts |
Thought to occur mainly in tropical areas: Africa, Asia, Middle East, and western Pacific |
As above |
Signs resemble fascioliasis caused by F hepatica |
|
Fasciolopsiasis |
Fasciolopsis buski |
Swine, people are definitive hosts; snails are intermediate hosts |
Asian pig-raising regions |
Ingestion of aquatic vegetables or contaminated drinking water containing metacercariae |
Often asymptomatic; gastroenteritis; intestinal bleeding, obstruction, or perforation possible; facial, abdominal, extremity edema may occur |
|
Gastrodiscoidiasis |
Gastrodiscoides hominis; uncertain whether people and swine carry the same strains |
Swine, people, nonhuman primates, rodents, other mammals are definitive hosts; snails are intermediate hosts |
Asia (including the Philippines), also reported in Africa, Volga delta in Russia |
Possibly ingestion of water or aquatic plants |
Mild diarrhea if high parasite burden |
|
Heterophyiasis |
Heterophyes spp, Haplorchis spp, other heterophids |
Cats, dogs, foxes, wolves, cattle, other mammals, fish-eating birds are definitive hosts (host varies with species of parasite); fish (and snails) are intermediate hosts |
Middle East (especially Nile delta), Turkey, Asia |
Ingestion of undercooked fish containing encysted larvae |
Diarrhea with mucus, colicky pain; heart or CNS involvement possible; severity of signs may vary with species |
|
Metagonimiasis |
Metagonimus yokogawai, M miyatai, M takahashii, and other Metagonimus spp |
Cats, dogs, rats, other fish-eating mammals, possibly birds are definitive hosts; fish (and snails) are intermediate hosts |
Human illness mainly in Asia, also reported in Siberia; parasites have been found in Europe |
Ingestion of undercooked freshwater fish containing encysted larvae |
Diarrhea with mucus, anorexia, mild epigastric pain or abdominal cramps; malabsorption, weight loss if high parasite burden |
|
Metorchiasis |
Metorchis conjunctus, Canadian liver fluke |
Dogs, foxes and other canids, cats, raccoons, muskrats, mink, other fish-eating mammals are definitive hosts; fish (and snails) are intermediate hosts |
North America; human infection rare |
Ingestion of undercooked freshwater fish containing encysted larvae |
Fever, abdominal pain (mainly epigastric), anorexia during acute stage; effects of chronic infection uncertain |
|
Nanophyetiasis |
Troglotrema salmincola (also called Nanophyetus salmincola) |
Raccoons, foxes, dogs, cats, skunks, and other fish-eating mammals and birds are definitive hosts; salmonid and some non-salmonid fish (and snails) are intermediate hosts |
North America along Pacific coast, Russia |
Ingestion of undercooked fish or roe |
Mild gastroenteritis |
|
Opisthorchiasis |
Opisthorchis felineus (cat liver fluke) |
Cats, dogs, foxes, swine, seals, other fish-eating mammals are definitive hosts; fish (and snails) are intermediate hosts |
Europe, Kazakhstan, Russia, Ukraine |
Ingestion of undercooked freshwater fish containing encysted larvae |
Acute febrile illness with arthralgia, lymphadenopathy, skin rash; suppurative cholangitis and liver abscess in subacute, chronic stages; possible increased risk of cholangiocarcinoma |
|
|
O viverrini (small liver fluke); zoonotic transmission can occur, but people are important hosts |
People, dogs, cats, rats, pigs, fish-eating mammals are definitive hosts; fish and snails are intermediate hosts |
Southeast Asia |
Ingestion of undercooked freshwater fish containing encysted larvae |
Upper abdominal pain, diarrhea, fever, jaundice possible acutely; chronic infections with cirrhosis, pancreatitis, high incidence of cholangiocarcinoma |
|
|
Amphimerus pseudofelineus |
Various mammals, birds, reptiles are definitive hosts; fish suspected as intermediate hosts |
North and South America |
Undetermined but probably ingestion of undercooked fish |
|
|
Paragonimiasis (Lung fluke disease) |
Paragonimus westermani, P heterotremus, P africanus, P mexicanus, and other species |
Dogs, cats, swine, wild carnivores, opossums, and other mammals are definitive hosts; snails and freshwater crustaceans are intermediate hosts; wild boars, sheep, goats, rabbits, birds, other animals are paratenic hosts |
Flukes are worldwide (distribution varies with species); most human infections in Asia, Africa, tropical America |
Ingestion of undercooked, infected freshwater crustaceans (crabs, crayfish); metacercariae on contaminated hands, fomites after preparing crustaceans, or undercooked meat from paratenic hosts such as wild boars |
Chills, fever possible during migration to lungs; pulmonary disease resembling tuberculosis with cough, blood-tinged sputum; abdominal form with dull pain, tenderness, possibly diarrhea; less often, neurologic signs, migratory skin nodules, other organ-specific symptoms; predominant signs vary with species of fluke |
|
Schistosomiasis, intestinal and hepatic |
Schistosoma japonicum |
Many mammals, including cattle, water buffalo (important host in Asia), swine, dogs, cats, deer, horses, nonhuman primates, and rodents, are definitive hosts; snails are intermediate hosts |
Asia |
Penetration of unbroken skin by cercariae from infected snails in water |
Acute disease can include urticarial rash, mild signs, isolated pulmonary signs, or Katayama syndrome (occurs especially after first infection; febrile illness, sometimes with cough, diarrhea, abdominal pain, hepatosplenomegaly, and/or rash/urticaria); apparent clinical recovery may be followed by chronic intestinal schistosomiasis with abdominal pain/discomfort, diarrhea ± blood; chronic hepatic schistosomiasis with hepatosplenomegaly followed by liver fibrosis, ascites, portal hypertension with hematemesis and/or melena, portocaval shunting with pulmonary signs; ectopic parasites can cause seizures, paralysis, meningoencephalitis; intestinal and hepatic lesions tend to progress rapidly; death can occur |
|
|
S mansoni |
People, nonhuman primates are major reservoir (definitive) hosts; also in rodents, insectivores, cattle, dogs; snails are intermediate hosts |
Africa, Middle East, South America, Caribbean |
Penetration of unbroken skin by cercariae from infected snails in water |
Acute disease in some; intestinal (most often) and/or hepatic schistosomiasis similar to S japonicum but not as rapidly progressive; glomerulonephritis a possible complication; ectopic CNS parasites tend to cause transverse myelitis; also causes genital schistosomiasis with reproductive problems; death can occur |
|
|
S mattheei; S bovis and S margrebowiei might also be zoonotic |
Definitive hosts are artiodactylid ruminants (cattle, sheep, goats, waterbuck, wildebeest, antelope, buffalo), also found in nonhuman primates; snails are intermediate hosts |
Southern Africa; seems to be rare in people, and some infections may have been misidentified |
Penetration of unbroken skin by cercariae from infected snails in water |
Suggested agent in intestinal and hepatic schistosomiasis |
|
|
S mekongi |
People are reservoir (definitive) hosts; also found in dogs, pigs; snails are intermediate hosts |
Southeast Asia |
Penetration of unbroken skin by cercariae from infected snails in water |
Acute disease absent or very rare; intestinal and hepatic schistosomiasis; death can occur |
|
|
S intercalatum, S guineensis |
Primarily people, rodents may also be definitive hosts; some other mammals, including nonhuman primates, susceptible to infection; snails are intermediate hosts |
Africa |
Penetration of unbroken skin by cercariae from infected snails in water |
Intestinal schistosomiasis only, often mild or asymptomatic; occasionally bloody feces, diarrhea |
|
Schistosomiasis, urinary |
S haematobium |
People are main reservoir (definitive host); occasionally infects nonhuman primates, pigs, buffalo, sheep, rodents, or other mammals; snails are intermediate hosts |
Africa (including Madagascar, Mauritius), the Middle East |
Penetration of unbroken skin by cercariae from infected snails in water |
Acute disease in some; chronic disease—hematuria, dysuria, kidney failure; calcification of bladder wall, ureter, and bladder can lead to bladder cancer; ectopic CNS parasites tend to cause transverse myelitis; genital schistosomiasis; death can occur |
|
Swimmer’s itch (Cercarial dermatitis) |
Schistosome cercariae from Schistosoma spp (mammals); Gigantobilharzia, Trichobilharzia, and Austrobilharzia spp (birds) |
Birds, mammals are definitive hosts; snails are intermediate hosts |
Worldwide |
Penetration of unbroken skin by cercariae from infected snails in fresh- and saltwater |
Self-limiting urticaria, pruritus, rash; fever, local lymph node swelling possible in some cases |
|
|
|||||
|
|
|||||
|
Parasitic Diseases—Cestodes (Tapeworms) |
|||||
|
Bertielliasis |
Bertiella studeri, B mucronata |
Nonhuman primates are usual hosts; other mammals, including dogs, people can be infected |
Asia, South America, Africa; can occur in imported primates in other areas |
Ingestion of infected oribatid mites in food |
Most cases asymptomatic; abdominal pain, vomiting, diarrhea, constipation, weight loss |
|
Coenuriasis (Coenurosis) |
Taenia multiceps |
Definitive hosts are canids; intermediate hosts are sheep, other herbivores |
Worldwide in scattered foci; mainly reported from Europe, Asia |
Ingestion of tapeworm eggs in canine feces, may be via water, vegetables, soil |
Painless skin swelling; possible CNS involvement (signs of mass lesion in brain) or larva in eye |
|
|
T serialis |
Definitive hosts are canids; intermediate hosts are lagomorphs, rodents, occasionally other mammals |
Africa, Europe, North America, Australia; rare in people |
As above |
Painless skin swelling; also in muscles and retroperitoneally; CNS involvement possible |
|
|
T brauni |
Definitive hosts are canids; intermediate hosts are gerbils, wild rodents, also people |
Africa |
As above |
Most often in subcutaneous tissues (skin swelling) or eye, also CNS |
|
Cysticercosis |
Taenia solium (see also Taeniasis) |
People are definitive hosts; swine, other mammals are intermediate hosts (people can be both definitive and intermediate hosts) |
Worldwide where swine are reared; most cases seen in Africa, Asia, Central and South America |
Ingestion of eggs (including autoinfection from adult parasite in human intestine) |
Inflammation in CNS caused by death of small larva or growth to large size (often years after infection); can cause seizures, other CNS signs; less often in eye or heart; massive numbers in muscles can also be symptomatic |
|
|
T crassiceps |
Foxes, also other canids and carnivores, including dogs, are definitive hosts; rodents, insectivores, rabbits, occasionally other mammals are intermediate hosts |
North America, Europe, Asia, and other areas where foxes are present |
Ingestion of eggs |
Tissue invasion (mainly subcutaneous, muscle), ocular; one paravertebral pseudohematoma with local bleeding, one CNS larva; many but not all cases in immunocompromised |
|
Diphyllobothriasis (Fish tapeworm infection) |
Diphyllobothrium latum (Dibothriocephalus latus), D nihonkaiense, D pacificum, D dendriticum, and other Diphyllobothrium spp |
Dogs, bears, seals, sea lions, gulls, and other fish-eating mammals and birds are definitive hosts; freshwater or marine fish (and copepods) are intermediate hosts |
Worldwide; distribution of species varies |
Ingestion of undercooked infected fish |
Usually asymptomatic; may cause mild abdominal distress, diarrhea (chronic relapsing diarrhea possible in some cases) |
|
Dipylidiasis (Dog tapeworm infection) |
Dipylidium caninum |
Dogs, cats, wild canids, some other wild carnivores are definitive hosts; fleas are intermediate hosts |
Worldwide; uncommonly reported in people |
Ingestion of dog or cat fleas |
Usually in children; asymptomatic or mild abdominal distress, diarrhea; proglottids in feces resemble cucumber seeds |
|
Echinococcosis |
Echinococcus granulosus sensu lato |
Dogs, other canids, hyenas are definitive hosts; sheep, goats, cattle, water buffalo, swine, camels, cervids, rodents, other mammals, or marsupials are intermediate or aberrant hosts; strains of parasite can be adapted to different intermediate hosts |
Worldwide, strains differ in distribution |
Ingestion of tapeworm eggs in food or water, to mouth on hands; eggs stick to fur and hands |
Cause space-occupying lesions of organs, especially lung, liver, also other organs, rarely CNS; cyst grows slowly, can cause death if untreated; rupture can cause allergic reactions, dissemination of cysts |
|
|
E multilocularis |
Foxes and other wild canids and felids are usual definitive hosts, but parasite can also mature in dogs, cats; intermediate hosts are usually rodents, insectivores, some other mammals |
North America (mainly Canada to north central USA), northern and central Eurasia |
Ingestion of tapeworm eggs in food or water, to mouth on hands; eggs stick to fur and hands |
Usually involves liver with mass lesions, occasionally lung or CNS; primary lesion can metastasize to many organs; without treatment, 70%–100% cases are fatal |
|
|
E oligarthrus |
Wild felids are definitive hosts, can mature in cats; agouti, pacas, spiny rats are intermediate hosts |
Central and South America; rare in people |
Ingestion of tapeworm eggs in food or water, to mouth on hands; eggs stick to fur and hands |
Has been seen in a variety of internal organs, eyes |
|
|
E vogeli |
Bush dogs are usual definitive host, can mature in other canids, including dogs; pacas, agouti, nutria, nonhuman primates, and other mammals can be intermediate hosts |
Central and South America |
Ingestion of tapeworm eggs in food or water, to mouth on hands; eggs stick to fur and hands |
Usually involves liver, may invade adjacent tissues; mortality high in advanced cases, even with treatment (22% in one study) |
|
Hymenolepiasis |
Hymenolepis nana (dwarf tapeworm); most human infections probably transmitted from people, but zoonoses possible |
People, nonhuman primates, rodents are definitive hosts; insects, including fleas, flour beetles, cereal beetles are intermediate hosts |
Worldwide |
Accidental ingestion of tapeworm eggs or infected insects; autoinfection possible |
Mainly in children; mild abdominal distress, decreased appetite, irritability are most common; weight loss, flatulence, diarrhea possible |
|
|
H diminuta (mouse tapeworm, rat tapeworm) |
Rats, mice are definitive hosts; insects, including fleas and cereal beetles are intermediate hosts |
Worldwide |
Ingestion of infected insects in food |
Mild abdominal symptoms of short duration |
|
Inermicapsifer infection |
Inermicapsifer spp |
Rodents, people are definitive hosts in Africa; people may be exclusive host outside Africa |
Africa, southeast Asia, tropical America |
Probably ingestion of infected arthropods |
Mild abdominal symptoms, if any |
|
Raillietina infection |
R celebensis, R demerariensis; most Raillietina spp have not been reported in people |
Rodents, nonhuman primates are definitive hosts for R celebensis, R demerariensis; other species in birds, mammals; arthropods, including ants, are intermediate hosts |
R demerariensis in tropical America (human cases mainly Ecuador, Cuba, Guyana, Honduras); R celebensis in Asia, Australia, Africa |
Probably ingestion of infected arthropods in food |
Vague discomfort, many cases asymptomatic; gastroenteritis, possibly other signs; mainly in children |
|
Sparganosis |
Spirometra spp (pseudophyllidean tapeworms, second larval stage) |
Dogs, cats, wild canids and felids are definitive hosts; copepods are first intermediate host; fish, frogs, reptiles are second intermediate hosts; primates, pigs, weasels, rodents, insectivores, other mammals, birds are paratenic hosts |
Worldwide; human cases most common in Asia |
Ingestion of infected cyclops (in water) or undercooked intermediate or paratenic host; application of contaminated tissues to skin (eg, as poultice) |
Nodular, itchy skin lesions that can migrate; conjunctival and eyelid lesions; urticaria, painful edema; other organ involvement, including CNS, eye |
|
Taeniasis |
|
|
|
|
|
|
—Asian taeniasis |
Taenia asiatica (also called T taiwanensis, T saginata asiatica |
Domestic and wild pigs, occasionally cattle, goats, monkeys are intermediate hosts; people are definitive hosts |
Asia |
Ingestion of undercooked animal products, usually visceral organs such as liver and lung |
Vague abdominal complaints and proglottid passage; anal pruritus; possible that ingestion of eggs may be followed by larval migration and disseminated disease (uncertain/controversial) |
|
—Beef tapeworm disease |
T saginata |
Cattle, water buffalo, llamas, reindeer, camels, other domestic and wild ruminants are intermediate hosts; people are definitive host |
Worldwide |
Ingestion of undercooked meat containing larvae |
Mild abdominal discomfort and proglottid passage; gravid proglottids may travel to ectopic sites and cause symptoms; eggs do not cause disseminated disease |
|
—Pork tapeworm disease; cysticercosis and neurocysticercosis |
T solium |
People are definitive host; swine, occasionally other mammals, including people, are intermediate hosts |
Worldwide where swine are reared; most cases seen in Africa, Asia, Central and South America |
Ingestion of undercooked pork containing larvae causes taeniasis; ingestion of eggs (including autoinfection from adult worm in intestine) causes cysticercosis |
Adult stage in intestine (taeniasis) mild or asymptomatic; cysticercosis usually asymptomatic for years until cysticercus becomes large or death of small cysticerci result in inflammation in CNS (seizures, other CNS signs) or infrequently in eye or heart; massive numbers in muscles can also be symptomatic |
|
|
|||||
|
|
|||||
|
Parasitic Diseases—Nematodes (Roundworms) |
|||||
|
Angiostrongyliasis |
Angiostrongylus costaricensis, also called Parastrongylus costaricensis |
Cotton rats and other rodents are definitive hosts; slugs are intermediate hosts |
Mainly in Central and South America, Caribbean parasite has also been reported in North America |
Accidental ingestion of slugs or possibly plants contaminated by their secretions |
Acute abdominal angiostrongyliasis; severe pain resembles appendicitis, especially in children; rarely, more insidious disease with liver involvement; complications can include intestinal ischemia, perforation; fatalities possible |
|
|
Angiostrongylus cantonensis, also called Parastrongylus cantonensis |
Rodents (rats, including Rattus and Bandicota spp) are definitive hosts; snails, slugs are intermediate hosts; land planarians, crustaceans (crabs, shrimp, prawns), amphibians, fish, reptiles are paratenic hosts |
Originated in Asia, spread to many other regions, mainly tropics, including Americas, Caribbean, Middle East, Australia |
Ingestion of raw/undercooked intermediate or paratenic host (or accidental ingestion on vegetables); possibly ingestion of plants contaminated by secretions of intermediate host |
Eosinophilic meningitis or meningoencephalitis, spinal cord involvement; ocular involvement with decreased vision; transient abdominal pain, pruritus in some; most cases relatively mild and self-limiting, but some fatal |
|
Anisakiasis |
Anisakis, Pseudoterranova, and Contracaecum spp |
Marine mammals (cetaceans and pinnipeds) and fish-eating birds are definitive hosts; fish, crustaceans, and cephalopod mollusks are intermediate or paratenic hosts |
Worldwide but many cases in northern Asia and western Europe |
Ingestion of undercooked marine fish, squid, octopus |
Gastroenteritis with upper quadrant pain; parasite usually in stomach; small-intestinal infections unusual but can occur; colon, esophagus rarely involved; oropharyngeal worm can cause hematemesis, cough; urticaria and other allergic signs after ingestion of live or dead worms |
|
Ascariasis |
Ascaris suum; potentially zoonotic (controversial) |
Pigs, also reported occasionally in other mammals, including nonhuman primates, sheep, cattle |
Worldwide, prevalence varies |
Ingestion of eggs from environment (shed in feces) |
Visceral larva migrans (respiratory signs, fever during larval migration); GI signs |
|
Capillariasis |
|
|
|
|
|
|
—Hepatic capillariasis |
Capillaria hepatica (also called Calodium hepaticum) |
Rodents major host, also in many other wild and domestic mammals |
Worldwide in scattered foci |
Ingestion of embryonated eggs in soil |
Acute or subacute hepatitis with marked eosinophilia; subclinical to fatal |
|
—Intestinal capillariasis |
C philippinensis (also called Paracapillaria philippinensis) |
Aquatic birds, people can be definitive hosts; freshwater fish are intermediate host |
Philippines, Thailand, also reported occasionally in other parts of Asia, Middle East, Cuba |
Ingestion of undercooked infected fish |
Enteropathy with protein loss and malabsorption; diarrhea, abdominal pain; weight loss can be severe; death possible |
|
—Pulmonary capillariasis |
C aerophila (also called Eucoleus aerophilus) |
Dogs, cats, other carnivores |
Worldwide; rare in people |
Accidental ingestion of infective eggs in soil or contaminated food |
Fever, cough, bronchospasm, bronchitis, dyspnea; can mimic bronchial carcinoma |
|
Dioctophymosis (Giant kidney worm infection) |
Dioctophyma renale |
Mink, dogs, and other carnivores are definitive hosts; annelids are intermediate hosts; frogs, fish are paratenic hosts |
Worldwide; rare in people |
Ingestion of infected fish, frog, or annelid |
Renal colic, hematuria, pyuria, ureteral obstruction, various kidney complications can be fatal; subcutaneous nodule |
|
Dracunculiasis (Guinea worm infection) |
Dracunculus medinensis; people are most important host but possible role for zoonotic transmission in some locations |
People, nonhuman primates are definitive hosts; infections have also been reported in animals, but parasite identification sometimes uncertain; domestic animals not thought to maintain parasite but possible exceptions (eg, dogs in Chad); copepods are intermediate hosts |
Asia (mainly Indian subcontinent) and Africa |
Ingestion of infected cyclops in water |
No symptoms until just before larviposition (~1 yr); papule to vesicular skin lesion to ulcer that opens in water to reveal worm; allergic reaction common at this time, and secondary infection may occur |
|
Filariasis |
|
|
|
|
|
|
—Dirofilariasis |
Dirofilaria immitis |
Dogs, cats, other mammals especially carnivores, mustelids, primates are definitive hosts (mainly patent in dogs and wild canids); mosquitoes are intermediate hosts |
Worldwide |
Bite of infected mosquitoes |
Fever, cough acutely, larvae result in infarct or coin lesion in the lungs; often asymptomatic; rarely involves eye or other body sites |
|
|
D tenuis, D repens, rarely other species |
D tenuis in raccoons; D repens mainly patent in dogs and some wild canids (eg, foxes); also infects cats but not usually patent |
D tenuis in North America; D repens in Asia, Europe, Africa |
Bite of infected mosquitoes |
Subcutaneous nodule or submucosal swelling, some migratory and/or painful; subconjunctival (rarely intraocular); internal location (mainly lung but also brain, other organs) possible |
|
—Malayan filariasis |
Brugia malayi; subperiodic form is of uncertain origin, thought to be zoonotic or maintained in both animals and people; periodic form is exclusive to people |
Cats, wild felids, pangolins, other carnivores, nonhuman primates susceptible |
Asia; subperiodic form limited to peninsular Malaysia, Thailand, and parts of Indonesia, Vietnam, and the Philippines in swamp-forest environments |
Bite of infected mosquitoes, Mansonia spp mainly associated with subperiodic form |
Lymphatic filariasis: recurrent painful lymphadenitis, lymphangitis, often preceded by prodromal illness with malaise or urticaria; may progress to elephantiasis, usually of legs; hypersensitivity syndrome with cough, chest pain, asthmatic attacks especially at night |
|
Filariasis caused by other Brugia species |
Brugia spp other than B malayi, including B pahangi |
Various domestic and wild mammals, including dogs and cats, are definitive hosts |
Asia, Africa, Americas |
Mosquitoes |
Occasional zoonotic infections (eg, cutaneous nodules, granuloma in lymph nodes) |
|
Gnathostomiasis |
Gnathostoma spinigerum, G binucleatum, and some other Gnathostoma spp |
Dogs, cats, wild carnivores are definitive hosts (G doloresi and G hispidum in pigs and wild boars); copepods, freshwater fish, eels, frogs, snakes, chickens, snails, pigs are intermediate or paratenic hosts |
Worldwide; most human cases from Asia; emerging along Pacific coast of Mexico, Ecuador, Peru, Argentina |
Ingestion of undercooked fish, poultry, or other intermediate or paratenic host, drinking water contaminated with copepods containing larvae; handling meat that contains larvae |
Fever, malaise, gastroenteritis, urticaria, soon after ingestion; migratory skin lesions (intermittent swelling, often painful or pruritic, or linear erythematous lesions) after weeks to years; may involve viscera, eye, or CNS; CNS involvement can be fatal or result in permanent damage with reported case fatality rates of 7%–25% |
|
Gongylonemiasis |
Gongylonema pulchrum |
Ruminants, domestic and wild swine, other mammals, birds are definitive hosts; coprophagous insects (eg, beetles, cockroaches) are intermediate hosts |
Worldwide; rare in people |
Ingestion of infected beetles, probably on vegetables; possible inhalation of small beetles |
Movement of parasite in submucosa of mouth is sensed; local irritation; pharyngitis, stomatitis possible |
|
Larva migrans, cutaneous (see alsognathostomiasis, above) |
Ancylostoma braziliense, A caninum, A ceylanicum, Uncinaria stenocephala |
Cats, dogs, wild carnivores are definitive hosts |
Worldwide; distribution varies with the species |
Contact with infective larvae that penetrate skin, usually via soil |
Itchy, serpiginous, migrating skin lesions; papules, nonspecific dermatitis, vesicles; wheezing, cough, and urticaria may occur; myositis or ocular lesions possible; eosinophilic enteritis after ingestion of A caninum; A ceylanicum can also become patent in intestine, causing GI signs, anemia |
|
|
Bunostomum phlebotomum |
Cattle |
Temperate regions |
As above |
As above |
|
|
Strongyloides stercoralis and other Strongyloides spp found in animals |
S stercoralis in dogs, cats, primates, including people; other species in swine, sheep, goats, cattle, horses, raccoons, and other domestic and wild mammals |
Worldwide, more common in tropics and subtropics |
Contact with infective larvae that penetrate skin, from soil or direct contact with feces; autoinfection possible with S stercoralis |
Larva currens (linear, serpiginous urticarial inflammation, often rapidly progressive); S stercoralis may also mature in intestine, causing enteritis and other signs (see below) |
|
Larva migrans, visceral (see alsoangiostrongyliasis and anisakiasis, above) |
Toxocara canis, T cati, possibly others |
Dogs and wild canids (T canis), cats and wild felids (T cati) are definitive hosts; many species can be paratenic hosts |
Worldwide |
Ingestion of embryonated eggs shed in feces of dogs and cats; via soil, water, food, fomites |
Fever, wheezing cough, upper abdominal discomfort; other symptoms, including neurologic signs, skin rashes also possible; may wax and wane for months; eye involvement (ocular migrans) may resemble retinoblastoma |
|
|
Baylisascaris procyonis, possibly other species of Bayliscascaris |
Raccoons, kinkajous are definitive hosts; dogs can be definitive or intermediate host; many mammals (including people), marsupials, and birds are intermediate or paratenic hosts |
North America, Europe, Japan |
Accidental ingestion of embryonated eggs in soil, water, or fecal-contaminated material |
Nonspecific signs, including fever, lethargy; hepatomegaly, pneumonitis, parasitic meningoencephalitis (may be fatal in infants, young children), ocular disease; other syndromes, including cardiac disease |
|
Oesophagostomiasis, Ternidensiasis |
Oesophagostomum spp, Ternidens deminutus; zoonotic potential may vary with parasite species/strain and geographic area |
Primates, including people |
Parasites found in Africa, Asia, South America; human cases mainly reported in Africa |
Ingestion of infective larvae in soil, often in food or water |
Abdominal pain and one or more masses ± mild fever; intestinal obstruction or abscessation possible; multinodular form (less common) with abdominal pain, persistent diarrhea, weight loss; rarely ectopic in omentum, liver, or skin |
|
Onchocercosis |
Onchocerca gutturosa, O cervicalis, O jakutensis, O dewittei japonica, O reticulata, O lupi, others |
Definitive hosts include cattle, horses, cervids, wild boars, dogs and other canids, camels, other species |
Distribution varies with species |
Probably transmitted by black flies (Diptera: Simuliidae), possibly other vectors |
Ocular disease, subcutaneous nodules |
|
Strongyloidiasis |
Strongyloides stercoralis; most human infections thought to be from strains adapted to people; frequency of maturation of canine S stercoralis in people undetermined, thought to be rare |
S stercoralis in dogs, cats, foxes, primates, including people |
S stercoralis worldwide; more common in tropical and subtropical climates |
Contact with infective larvae that penetrate skin, in soil or direct contact with feces; autoinfection possible |
Frequently asymptomatic in healthy; possible larva currens (seelarva migrans, above); respiratory signs in some (cough to bronchopneumonia), especially in elderly, immunocompromised; abdominal pain, diarrhea, sometimes with periodic urticarial or maculopapular rash; disseminated strongyloidiasis, neurologic complications, septicemia, and death may occur in immunocompromised |
|
|
S fuelleborni |
Primates, including people |
Africa, Asia, and in captive primates in other areas |
As above |
Associated with abdominal pain, occasional diarrhea, not well studied |
|
Thelaziasis (Eyeworms) |
Thelazia callipaedia, T californiensis, possibly T rhodesii |
Definitive hosts are dogs and other canids, cats (T callipaedia); dogs, wild mammals, occasionally cats (T californiensis); flies are intermediate hosts |
T callipaedia in Asia, Europe; T californiensis in North America (western USA); rarely in people |
Flies release parasite larvae on conjunctiva |
Conjunctivitis; corneal scarring, opacity in chronic cases |
|
Trichinosis (Trichinellosis) |
Trichinella spiralis and subspecies, T nativa, T britovi, T nelsoni, T pseudospiralis, possibly others |
Main reservoir may be wild carnivores (foxes, badgers, wolves, lynx), omnivores (bears, boars); also in any mammal that eats (or is fed) meat, including domestic swine, rodents, cats, dogs, horses, marine mammals; also birds (T pseudospiralis); T zimbabwensis (zoonotic potential unknown) can infect reptiles |
Worldwide, especially in temperate regions; some species are limited in their distribution |
Ingestion of undercooked pork, horse meat, game, and other tissues containing viable cysts |
Gastroenteritis in some; followed by fever, headache, severe myalgia, facial swelling (especially eyelids); ocular pain, rashes, or pruritus possible; pneumonitis, CNS, or myocardial involvement can occur; inapparent to fatal |
|
Trichostrongyliasis |
Trichostrongylus spp |
Cattle, sheep, other domestic and wild ruminants, sometimes other mammals |
Worldwide |
Ingestion of infective larvae on vegetables or in contaminated water, soil |
Asymptomatic or mild gastroenteritis |
|
Trichuriasis (Whipworm infection) |
Trichuris suis, possibly T vulpis and other species; main species in people is T trichiura, but zoonotic infections are unusual |
T vulpis in canids; T suis in domestic and wild swine |
Worldwide, especially warm, humid climates |
Ingestion of embryonated eggs on plant foods, water, or in soil |
T suis can colonize people, who develop GI signs; rare larva migrans or intestinal infections suggested from T vulpis (controversial identification) |
|
|
|||||
|
|
|||||
|
Parasitic Diseases—Acanthocephalans |
|||||
|
Acanthocephaliasis, Macracanthorhynchosis |
Macracanthorhynchus hirudinaceus and other species |
Hosts vary with parasite species; definitive hosts include domestic and wild pigs, rodents, muskrats, arctic foxes, dogs, sea otters, other terrestrial and marine mammals; intermediate hosts are beetles, cockroaches, crustaceans; fish are paratenic hosts |
Worldwide |
Ingestion of infected beetles, other intermediate hosts, or fish |
Gastroenteritis, may lead to gut perforation or intestinal obstruction; some cases asymptomatic |
|
|
|||||
|
|
|||||
|
Parasitic Diseases—Annelids (Leeches) |
|||||
|
Hirudiniasis (internal) |
Limnatis nilotica and other aquatic leeches |
Cattle, buffalo, other domestic and wild mammals, probably frogs |
Africa, Asia, southern Europe, Middle East |
Drinking unfiltered water (leech enters nares or mouth), wading in deep water (enters genitourinary tract) |
Attaches to nasopharynx, pharynx, esophagus, occasionally deeper in respiratory tract, or in genitourinary tract; pressure and/or pain at attachment site; bleeding (eg, hemoptysis, hematemesis, epistaxis, vaginal bleeding), anemia (can be severe); other signs depend on location |
|
|
|||||
|
|
|||||
|
Arthropod Diseases |
|||||
|
Acariasis (Mange) |
Mites of Sarcoptes, Cheyletiella, Dermanyssus, and Ornithonyssus spp, Notoedres cati, Trixacarus caviae, Liponyssoides sanguineus; possibly others (uncommon) |
Mammals and birds |
Worldwide |
Contact with infected animals, fomites |
Itchy skin lesions |
|
Myiasis |
Cochliomyia hominivorax and Chrysomya bezziana (screwworms) |
Mammals; rare in birds |
C hominivorax in South America, Caribbean; C bezziana in Asia, Africa, Middle East |
Flies lay eggs on host, larvae enter wounds (as small as a tick bite), mucous membranes |
Painful, pruritic, foul-smelling, enlarging dermal and subdermal wounds or nodules, often with serosanguineous discharge; some infestations in cavities, including nasal cavity; larvae can invade living tissue, locally destructive (including bone, eye, sinuses, or cranial cavity); can be fatal if untreated |
|
|
Cordylobia anthropophaga, rarely C rodhaini (Tumbu flies) |
Mammals, often found in dogs, rodents |
Africa, Middle East; also reported in Mediterranean region of Europe |
Larvae from environment invade unbroken skin |
Furuncular swelling at site of invasion, often feet; fever, malaise, focal lymphadenopathy possible |
|
|
Cuterebra spp |
Rodents, lagomorphs, occasionally other mammals |
North America |
Larvae from vegetation enter host in natural cavities or invade intact skin |
Subcutaneous furunculoid nodule(s); creeping skin eruption (uncommon); ocular lesions; rarely larvae might be found in upper respiratory tract |
|
|
Dermatobia hominis (human botfly) |
Mammals, some birds |
South and Central America, Mexico |
Eggs carried by other insects (eg, mosquitoes); larvae hatch and penetrate skin of mammalian host when insect lands |
Nonmigratory larvae in furuncles; episodes of pain, intense pruritus, sometimes with lymphangitis or lymphadenitis; can invade eyelids, eye sockets, mouth, especially in children |
|
|
Gasterophilus spp (equine botfly) |
Equids, occasionally other mammals |
Worldwide |
Accidental exposure to larvae |
Serpiginous, pruritic red stripes on skin resembling cutaneous larva migrans; very rarely might reach stomach (nausea, vomiting) |
|
|
Hypoderma lineatum, H bovis (warbles), and other Hypoderma spp |
H bovis and H lineatum in cattle, sometimes other mammals; other species primarily parasites of deer, caribou, or yaks |
North America, Europe, Asia; species distribution varies |
Eggs laid on host, larvae invade skin |
Usually subcutaneous (slowly moving furuncles that can appear and disappear) or similar to cutaneous larva migrans; endophthalmia uncommon; H lineatum may also cause an eosinophilic syndrome with fever, muscle pain, sometimes respiratory, cardiac, or neurologic signs |
|
|
Oestrus ovis, Rhinoestrus purpureus |
O ovis mainly in sheep, goats, also other mammals; R purpureus mainly in equids |
O ovis worldwide, usually in warmer climates; R purpureus in Asia, Africa, Europe |
Larvae are deposited in nares, conjunctiva, occasionally lips/mouth by adult fly |
Conjunctival form, with lacrimation and sensation of irritating foreign body in eye, ocular destruction rare; nasal form with localized pain or pruritus, congestion, headache; also reported in pharynx (inflammation, vomiting, dysphagia), rarely ear; usually self-limiting (except inside eye), because larvae cannot develop beyond first stage in people |
|
|
Wohlfahrtia spp, Wohlfahrtia vigil, W magnifica |
W vigil in rabbits, rodents, mink, foxes, dogs, and other carnivores, other mammals; W magnifica in sheep, cattle, dogs, other mammals, some birds, especially geese |
W vigil in North America; W magnifica in Europe (mainly Mediterranean), north Africa, Asia |
Larvae deposited on host or nearby, penetrate lesions (both agents) or intact skin (W vigil) and natural orifices |
W vigil causes subcutaneous abscesses, furuncles; W magnifica has been reported from skin, eye, vulva, ear, orotracheal region |
|
Pentastomid infections |
Armillifer spp (tongue worms) |
Definitive hosts are snakes; intermediate hosts are rodents and other wild animals |
Africa, Asia |
Ingestion, via water or vegetables contaminated with eggs (from feces or saliva of snakes); undercooked snake meat; contaminated hands, fomites after handling snake meat |
Usually asymptomatic; large numbers of parasites can cause multifocal abscesses, masses, or obstruction of ducts in internal organs; symptoms vary with location; death rare |
|
|
Linguatula serrata |
Definitive hosts are dogs and other canids, felids; intermediate hosts are herbivores (especially sheep, goats, lagomorphs) and people |
Worldwide |
Ingestion of water or vegetables contaminated with eggs (from feces, saliva, or nasal discharge of definitive host); ingestion of larvae in undercooked liver or lymph nodes from intermediate hosts |
Ingestion of eggs—usually asymptomatic; ocular or pulmonary signs, abdominal pain, icterus, and other symptoms possible from invasion of internal organs Ingestion of larvae—throat irritation, pain; edema, congestion of nasopharynx may cause dyspnea, difficulty swallowing; most severe cases are probably in people who have been sensitized |
|
Tick paralysis (see Tick Paralysis) |
More than 40 species of ticks are capable of causing this disease; Dermacentor andersoni, D variabilis most common in North America |
Various animals carry ticks |
Worldwide |
Tick attachment |
Ascending flaccid paralysis, may be preceded by prodromal flu-like illness (malaise, weakness); can cause respiratory paralysis, also paresthesia; ends when tick is removed |
|
Tunga infections |
Tunga penetrans (sand fleas, jiggers) |
People, dogs, pigs, other mammals |
Africa, Central and South America, Caribbean, south Asia |
Skin contact with contaminated soil |
Penetration of skin and burrowing result in pain and itching around discrete sores, often on feet; may be secondarily infected |
|
|
|||||
|
|
|||||
|
Viral Diseases |
|||||
|
Alkhurma virus infection |
Alkhurma virus (family Flaviviirdae, genus Flavivirus); may be a variant or strain of Kyasanur Forest virus |
Sheep, goats, camels |
Middle East, mainly reported in Saudi Arabia, also Egypt |
Ticks (Ornithodoros and Hyalomma spp); direct contact with animal meat via broken skin or ingestion of unpasteurized camel milk linked to some cases |
Febrile illness, often with GI signs (eg, vomiting, abdominal pain); encephalitic/neurologic and hemorrhagic signs in some; case fatality up to 25% in early reports, recently <1% |
|
Barmah Forest virus infection; epidemic polyarthritis |
Barmah Forest virus (family Togaviridae, genus Alphavirus) |
Natural hosts unknown; horses, brushtail possums may be hosts |
Australia |
Mosquito bites; Culex annulirostris and Aedes spp implicated |
Resembles disease caused by Ross River virus (see entry later in this table) but seems to persist longterm in fewer patients, rash more common |
|
Buffalopox virus infection |
Vaccinia virus, Buffalopox virus strain (family Poxviridae, genus Orthopoxvirus) |
Water buffalo, cattle |
Indian subcontinent (south Asia), Egypt, Indonesia |
Skin contact with infected animals, often when milking |
Pox skin lesions mainly on hands, face, legs, buttocks; occasionally lymphadenopathy, fever, malaise |
|
California encephalitis virus serogroup (California serogroup) infections |
California encephalitis virus serogroup (family Bunyaviridae, genus Orthobunyavirus); includes California, La Crosse, Tahyna, Inkoo, Jamestown Canyon, Morro Bay, Snowshoe hare, Guaroa, Lumbo, Chatanga, and other viruses |
Many wild and domestic mammals |
North and South America, Europe, Africa, Asia; possibly worldwide; distribution of each virus varies |
Mosquito bites |
Syndromes, severity vary with the virus; flu-like illness, meningitis, or encephalitis common with North American viruses |
|
—La Crosse encephalitis |
La Crosse virus (California encephalitis virus serogroup) |
Chipmunks, squirrels are major amplifying hosts; rabbits, foxes, and other mammals can be infected |
North America |
Mosquito bites |
Many cases mild and flu-like; meningitis or encephalitis with seizures, paralysis, and focal neurologic signs possible; most cases in children; estimated case fatality rate <1% in cases with encephalitis |
|
—Tahyna fever |
Tahyna virus (California encephalitis virus serogroup) |
Hares, rabbits, rodents, hedgehogs, and other mammals |
Europe, Asia, Africa |
Mosquito bites (culicine and anopheles) |
Influenza-like illness, sometimes including GI signs; arthritis or respiratory signs, including bronchopneumonia in some; meningitis possible; most often in children; does not appear to cause fatal disease |
|
Camelpox |
Camelpox virus |
Old World camelids, possibly other species |
Middle East, Asia, Africa, possibly other areas; human cases recently described in India in camel handlers, rare unconfirmed cases suggested in other locations |
Direct contact |
Skin lesions similar to cowpox, variola virus infections |
|
Chikungunya virus infection |
Chikungunya virus (family Togaviridae, genus Alphavirus) |
Sylvatic cycle in nonhuman primates, possibly rodents in Africa; virus thought to be maintained in people in Asia, but sylvatic cycle may also exist |
Asia, Africa |
Mosquito bites (especially Aedes spp) |
Febrile illness, may have rash and/or GI signs; arthralgia, especially in small joints, and myalgia prominent, may persist for months; myocarditis, neurologic signs, hemorrhages reported in a few cases |
|
Colorado tick fever |
Colorado tick fever virus (family Reoviridae, genus Coltivurus; Salmon River virus and California hare coltivirus may be variants |
Rodents; also found in porcupines, lagomorphs, deer, elk, and other mammals |
Rocky Mountain region of North America |
Tick bites (primary vector is Dermacentor andersoni) |
Nonspecific febrile illness; pharyngitis, rash, or GI signs possible; biphasic or triphasic in some; complications (eg, neurologic signs, hemorrhages, pericarditis, myocarditis, orchitis) uncommon but can occur in severe cases; deaths rare |
|
Contagious ecthyma (Orf, see Contagious Ecthyma) |
Orf virus (family Poxviridae, genus Parapoxvirus) |
Sheep, goats, camelids, reindeer, wild ungulates; rare cases in dogs |
Worldwide |
Occupational exposure via contact with broken skin (both live animals and meat processing) |
Papule(s) that umbilicate and ulcerate, usually on hands; dissemination rare; large lesions refractory to treatment can be seen in immunosuppressed |
|
Cowpox (see Pox Diseases) |
Cowpox virus (family Poxviridae, genus Orthopoxvirus) |
Rodents are usual reservoir host; also in domestic and wild cats, occasionally cattle, other mammals |
Parts of Europe and Asia |
Contact exposure via broken skin, bites, scratches |
Papules, vesicles that become pustular, to ulcerative nodules, scars; single or multiple lesions, often on hands; regional adenopathy and malaise, flu-like symptoms in some; lesions remain localized in healthy people; more extensive or generalized disease may be seen in children, people with eczema,immunocompromised; severe cases can involve respiratory mucosa; rare fatal cases (eg, complications of encephalitis) |
|
Crimean-Congo hemorrhagic fever (see Crimean-Congo Hemorrhagic Fever) |
Crimean-Congo hemorrhagic fever virus (family Bunyaviridae, genus Nairovirus) |
Cattle, rodents, sheep, goats, hares, other mammals, some birds |
Africa, Middle East, central Asia, southeastern Europe; appears to be spreading |
Tick bites, especially Hyalomma but also Rhipicephalus, Dermacentor, other species; skin contact with animal or human blood or tissues or crushed ticks; ingestion of unpasteurized milk |
Fever, headache, pharyngitis, abdominal symptoms, petechial rash, hemorrhage, hepatitis, other organ involvement in some cases; very severe in pregnant women; case fatality rate 3%–50%, varies with region |
|
Eastern equine encephalomyelitis (see Equine Arboviral Encephalomyelitis) |
Eastern equine encephalomyelitis virus (family Togaviridae, genus Alphavirus); North American lineage 1 strains more virulent than South American lineages |
Birds are principal reservoir hosts in North America, snakes might have role in overwintering virus; rodents, marsupials might be reservoir hosts in South America; clinical cases seen in equids and occasionally other mammals and birds; mammals are almost always dead-end hosts |
Western hemisphere |
Mosquito bites; Culiseta melanura important in maintenance cycle in birds in North America; various mosquito species (Aedes, Coquillettidia, Culex) can transmit to people |
Nonspecific febrile illness may be followed by severe encephalitis, especially with North American lineage; neurologic sequelae common after encephalitis; case fatality rate 30%–70% with North American lineage; more severe in infants and elderly |
|
Ebola hemorrhagic fever |
Zaire ebolavirus, Sudan ebolavirus, Ivory Coast ebolavirus, Bundibugyo ebolavirus (family Filoviridae, genus Ebolavirus); Reston ebolavirus does not seem to affect people |
Bats are reservoir hosts for Zaire ebolavirus and suspected reservoir hosts for others; primates, duikers, possibly other mammals can be infected |
Africa |
Contact with infected tissues (especially nonhuman primates and duikers); probable transmission from bats in caves |
Initially nonspecific febrile illness; maculopapular rash with desquamation; mild to severe bleeding tendency develops a few days after onset; mortality rate 36%–90%, varies with isolate |
|
Encephalomyocarditis |
Encephalomyocarditis virus (family Picornaviridae, genus Cardiovirus); thought to be zoonotic |
Rodents may be reservoir hosts; also in swine, nonhuman primates, elephants, other mammals, and wild birds |
Worldwide in animals |
Uncertain |
Nonspecific febrile illness, sometimes with GI signs, and/or decreased reflexes have been reported in adults, with recovery within several days; CNS signs, including paralysis, have been reported in children |
|
Foot-and-mouth disease (see Foot-and-Mouth Disease) |
Foot-and-mouth disease virus (family Picornaviridae, genus Aphthovirus, types A, O, C, SAT 1, SAT 2, SAT 3, and Asia 1) |
Cattle, swine, sheep, goats, other cloven-hoofed animals (Artiodactyla), a few mammals in other orders |
Asia, Africa, Middle East, South America |
Contact exposure, often in laboratories or other high concentrations of virus |
People may become temporary nasal carriers of virus but do not usually become ill; mild influenza-like disease with vesicular lesions occurs very rarely |
|
Hantaviral diseases |
|
|
|
|
|
|
—Hantaviral pulmonary syndrome |
Sin Nombre, Black Creek Canal, Bayou, Andes, Bermejo, Choclo, Araraquara, Juquitiba, Laguna Negra, and Castelo dos Sonhosviruses, others (family Bunyaviridae, genus Hantavirus) |
Rodents; each virus tends to be associated with a single reservoir host |
North and South America |
Aerosols from rodent excretions and secretions; contact with broken skin and mucous membranes; rodent bites |
Prodromal stage with nonspecific febrile illness; followed by respiratory failure, cardiac abnormalities; hemorrhagic signs possible with South American viruses; significant kidney disease uncommon; mortality rate varies with the virus, but can reach 40%–60% |
|
—Hemorrhagic fever with renal syndrome |
Hantaan virus, Dobrava virus, Puumala virus, Seoul virus, Saaremaa virus, others (family Bunyaviridae, genus Hantavirus) |
Rodents; each virus tends to be associated with a single reservoir host, but Seoul virus is carried by both Rattus norvegicus and R rattus |
Europe, Asia; Seoul virus is worldwide |
Aerosols from rodent excretions and secretions; contact with broken skin and mucous membranes; rodent bites |
Prodromal stage with abrupt onset of fever, headache, back pain, sometimes petechiae, GI signs (may be severe); followed by hypotension, renal signs to renal failure with oliguria; hemorrhage, other syndromes in some; mortality rate varies with the virus, from <1% (Puumala virus) to 10%–15% (Hantaan virus) |
|
Hendra virus infection (see Hendra Virus Infection) |
Hendra virus (family Paramyxoviridae, genus Henipavirus) |
Fruit bats are normal reservoir host; horses can be infected |
Australia |
Direct contact with infected animals (all human cases have been linked with horses) or contaminated tissues |
Respiratory infection, encephalitis (including recurrent encephalitis); few cases described but several were fatal |
|
Hepatitis E |
Hepatitis E virus, mammalian isolates (family Hepeviridae, genus Hepevirus); genotypes 3 and 4 zoonotic; genotypes 1 and 2 maintained in people |
People; animals, including swine, wild boar, deer, rabbits, ferrets, rats, mongoose, others; swine and probably other hosts are reservoirs for human infections |
Worldwide; human and zoonotic genotypes may differ in prevalence between areas |
Fecal-oral spread; consumption of raw or undercooked meat and liver; waterborne, contact with animal reservoirs |
Mild, self-limiting hepatitis to liver failure, more severe in pregnancy and can result in abortion, death of newborn, premature birth; usually acute, but can be chronic in organ-transplant patients; case fatality rate <1% to 4% in general population, up to 20% in pregnant |
|
Herpes B virus disease |
Cercopithecine herpesvirus 1 (McHV, Herpesvirus simiae, B virus) (family Herpesviridae, genus Simplexvirus) |
Carried in genus Macaca (Old World macaques), with lifelong latency and potential for periodic shedding after infection; other nonhuman primates susceptible; cell cultures |
Worldwide, can be common, especially in closed groups of macaques; human cases rare |
Monkey bites and scratches, contamination of mucous membranes with infected saliva, secretions |
Influenza-like symptoms; vesicular skin lesions, pain, or itching around wound, followed by severe encephalitis with seizures, paralysis, coma; 85% mortality rate |
|
Influenza virus infections |
|
|
|
|
|
|
—Avian influenza |
Influenza A virus (family Orthomyxoviridae, genus Influenzavirus A); avian influenza viruses; many severe human cases linked to Asian lineage H5N1 highly pathogenic avian influenza (HPAI) viruses, but other viruses also cause illness |
Avian influenza viruses in wild and domestic birds, especially poultry; uncommon in mammals |
Worldwide, distribution of strains varies |
Usually by contact with infected poultry; avian viruses may be shed in respiratory secretions and feces |
Avian influenza viruses can cause conjunctivitis, human influenza-like illness, or severe disease with multiorgan dysfunction, death; severity of disease varies with influenza strain |
|
—Swine influenza |
Influenza A virus (family Orthomyxoviridae, genus Influenzavirus A); swine influenza viruses |
Usually in pigs; also turkeys; can infect mink, ferrets |
Worldwide |
Usually by contact with infected animals; swine influenza viruses occur in respiratory secretions |
Seems to resemble human influenza; severity of disease varies; fatal cases have been reported uncommonly |
|
Japanese encephalitis (Japanese B encephalitis) |
Japanese encephalitis virus (family Flaviviridae, genus Flavivirus) |
Swine, wild birds are important maintenance hosts; horses ill but epidemiologically unimportant in amplification; other mammals, reptiles, amphibians may be infected, usually asymptomatically |
Asia, Australia, Papua New Guinea, Pacific islands from Japan to the Philippines |
Mosquito bites (Culex tritaeniorhynchus) important in maintenance cycle, other Culex and Aedes spp can transmit); also through broken skin or mucous membranes after direct contact with infected tissues |
Fever, chills, myalgia, severe headache, GI symptoms; can progress to severe encephalitis; neurologic sequelae very common in survivors of encephalitis; case fatality rate 15%–30% |
|
Kyasanur Forest disease |
Kyasanur Forest virus (family Flaviviridae, genus Flavivirus) |
Rodents, shrews, other small mammals might be reservoirs (uncertain); affects monkeys; possible infections in other mammals, birds |
India |
Tick bites (especially Haemaphysalis spinigera, also others) |
Nonspecific febrile illness; course may be biphasic; hemorrhagic signs (eg, ecchymoses, purpura, petechiae, GI bleeding, epistaxis) and/or neurologic signs possible in second stage; prolonged convalescence in many; case fatality rate ~3% |
|
Lassa fever |
Lassa virus (family Arenaviridae, genus Arenavirus) |
Wild rodents, usually multimammate mouse |
West Africa |
Contact with rodent excretions, secretions, or tissues; aerosols |
Gradual onset of nonspecific febrile illness, may be followed by chest pain, cough, GI signs, hepatitis; severe swelling of head and neck, hypotension/shock can develop; pleural/pericardial effusions; hemorrhagic syndrome less common; overall mortality rate 1% in endemic areas; case fatality rate 20% among hospitalized patients |
|
Louping ill (Ovine encephalomyelitis, see Louping Ill) |
Louping ill virus (family Flaviviridae, genus Flavivirus) |
Sheep, goats, also in llamas, cattle, horses, other domestic and wild mammals, grouse, ptarmigan |
UK, Northern Ireland; also reported in Norway, Spain; uncommon in people |
Tick bites (Ixodes ricinus); aerosol exposure in laboratory, contamination of skin wounds, contact with infected animals; possibly ingestion of milk |
Biphasic influenza-like illness, sometimes followed by meningitis or meningoencephalitis, paralysis, joint pain in second phase; not usually fatal |
|
Lymphocytic choriomeningitis |
Lymphocytic choriomeningitis virus (family Arenaviridae, genus Arenavirus) |
Reservoir mainly house mouse; can be maintained in some other mice, hamster populations; also infects guinea pigs, chinchillas, rats, nonhuman primates, some other mammals |
Worldwide |
Contact with host excretions and secretions; bites; possibly ingestion |
Ranges from mild flu-like illness to biphasic with meningitis in second phase; complications (eg, arthritis, parotitis, orchitis) possible; can cause congenital defects (CNS defects, chorioretinitis, and other ocular lesions) or abortion; rarely fatal in immunocompetent (overall case fatality rate <1%) |
|
Marburg hemorrhagic fever |
Marburg virus (family Filoviridae, genus Marburgvirus) |
Bats are reservoir hosts; primates can be infected |
Africa |
Contact with infected tissues (especially nonhuman primates); probable transmission from bats in caves |
Initially nonspecific febrile illness; maculopapular rash with desquamation; hepatitis; mild to severe bleeding tendency develops a few days after onset; mortality rate 20%–88%, varies with isolate |
|
Menangle virus infection |
Menangle virus (family Paramyxoviridae) |
Fruit bats are normal reservoir host; pigs can also be reservoir |
Australia |
Close direct contact with tissues, amniotic fluid or blood of pigs reported in human cases |
Severe illness with fever, severe headache, myalgia, lymphadenopathy, drenching sweats, macular rash |
|
Middle East respiratory syndrome (MERS) |
MERS coronavirus |
Unknown reservoir host, possibly bats; source of infection for people uncertain, camels implicated |
Middle East |
|
Pneumonia, more likely in people with coexisting illness or immunosuppression but also in healthy; ~50% of known cases were fatal |
|
Milker’s nodules (Pseudocowpox, see Pseudocowpox) |
Pseudocowpox virus (family Poxviridae, genus Parapoxvirus) |
Cattle |
Worldwide |
Skin contact (especially broken skin) with lesions on cow’s udder or mouth of calf; also from fomites |
Papular to nodular red skin lesions; self-limiting |
|
Monkeypox |
Monkeypox virus (family Poxviridae, genus Orthopoxvirus); Congo Basin clade causes more severe illness than West African clade |
Nonhuman primates, some wild and pet rodents, including Gambian rats, dormice, prairie dogs, African squirrels, some other mammals such as opossums; full host range uncertain |
West and central Africa |
Contact with lesions, blood or body fluids, fomites; bites; aerosols during close contact |
Smallpox-like disease; flu-like symptoms followed by maculopapular rash, which develops into vesicles, pustules, scabs; lymphadenopathy prominent; respiratory signs, encephalitis possible; case fatality rate varies with strain, <1% to 10%–17% or higher; milder in those vaccinated for smallpox |
|
Murray Valley encephalitis |
Murray Valley encephalitis virus (family Flaviviridae, genus Flavivirus) |
Wild water birds |
Australia, New Guinea |
Mosquito bites (Culex annulirostris) |
Asymptomatic or mild nonspecific febrile illness in majority; encephalitis, often with neurologic sequelae, or poliomyelitis-like flaccid paralysis in small number of patients; case fatality rate 15%–30% in encephalitic form |
|
Newcastle disease |
Newcastle disease virus/Avian paramyxovirus 1 (family Paramyxoviridae, genus Avulavirus) |
Domestic and wild birds |
Mildly virulent (lentogenic, mesogenic strains) are found worldwide; highly virulent (velogenic) strains found in Asia, the Middle East, Africa, Central and South America, parts of Mexico; also in cormorants in USA |
Occupational exposure, usually after contact with large amounts of virus |
Highly virulent (velogenic) strains can cause self-limiting conjunctivitis, possibly other syndromes |
|
New World hemorrhagic fever (Argentinean, Bolivian, Venezuelan and Brazilian hemorrhagic fevers [HF]) |
Arenaviruses in Tacaribe complex (family Arenaviridae, genus Arenavirus): Juin virus (Argentine HF), Machupo virus (Bolivian HF), Guanarito virus (Venezuelan HF), Sabiá virus (Brazilian HF), Chapare virus; possibly others |
Rodents |
South America, related viruses might exist among rodents in Mexico |
Viruses found in rodent excretions, secretions, tissues; inhalation of aerosolized virus or direct contact with mucous membranes or broken skin |
Gradual onset of nonspecific signs, including myalgia, headache, and fever; may develop petechial or ecchymotic hemorrhages, bleeding, CNS signs, hypotension/shock; case fatality rate in untreated Bolivian hemorrhagic fever 5%–30%, untreated Argentine hemorrhagic fever 15%–30% |
|
Nipah virus infection (see Nipah Virus Infection) |
Nipah virus (family Paramyxoviridae, genus Henipavirus) |
Fruit bats are normal reservoir; swine can be reservoir; occasionally in other mammals (spillover hosts) |
Malaysia, Bangladesh, and Northern India; virus is probably endemic in southeast Asia, but outbreaks seem to cluster in certain geographic areas |
Direct contact with infected pigs or contaminated tissue; direct or indirect (eg, contaminated fruit juice) bat-to-human transmission |
Initial signs flu-like with fever, headache, myalgia, sometimes vomiting; encephalitis and meningitis; respiratory disease, including acute respiratory distress syndromes in some; septicemia; other complications in severely ill; case fatality rate 33%–75% |
|
Omsk hemorrhagic fever |
Omsk hemorrhagic fever virus (family Flaviviridae, genus Flavivirus) |
Voles, muskrats; also found in other animals |
Siberia |
Tick bites (Dermacentor spp); direct contact with body fluids or carcasses of muskrats and possibly other animal hosts |
Biphasic febrile illness with headache, GI signs, ± hemorrhages (nose, gums, lungs, uterus); CNS signs in minority of patients; mortality rate <3% |
|
Powassan virus encephalitis |
Powassan virus (family Flaviviridae, genus Flavivirus); two closely related lineages in different reservoirs |
Rodents (groundhog, squirrels, mice) and other small mammals thought to be reservoirs |
North America, eastern Russia |
Ixodes spp ticks, also found in Dermacentor andersoni |
Nonspecific febrile illness; may progress to neurologic signs, which may be severe; some cases fatal |
|
Rabies and rabies-related infections (see Rabies) |
Lyssaviruses:rabies virus (family Rhabdoviridae, genus Lyssavirus) and the related lyssaviruses, Duvenhage virus, Mokola virus, Australian bat lyssavirus, European bat lyssaviruses 1 and 2, Irkut virus, possibly others |
Wild and domestic canids, Mustelidae, Viverridae, Procyonidae, and order Chiroptera (bats) are important reservoir hosts; all mammals are susceptible; bats are reservoir hosts for Duvenhage virus, Australian bat lyssavirus, and European bat lyssaviruses; Mokola virus carried in rodents and shrews |
Rabies is worldwide with some exceptions: completely absent from some islands; countries also considered rabies-free if no cases in people or domestic animals for 2 yr; rabies-related lyssaviruses found only in Eastern Hemisphere (distribution varies) |
Bites of diseased animals; aerosols in closed environments |
Paresthesias or pain at bite site; nonspecific prodromal signs such as fever, myalgia, malaise; mood changes progress to paresthesias, paresis, seizures, and many other neurologic signs; survival in clinical cases thought to be very rare |
|
Rift Valley fever (see Rift Valley Fever) |
Rift Valley fever virus (family Bunyaviridae, genus Flavivirus) |
Sheep, goats, cattle, buffalo, African buffalo, camels, nonhuman primates; squirrels and other rodents; puppies and kittens |
Africa, foci on Arabian peninsula, Indian subcontinent |
Mosquito bites (Aedes spp and Culex triteniorynchus); contact with tissues |
Influenza-like febrile illness in most; complications, including hemorrhagic fever, meningoencephalitis in <5%; ocular disease in 1%–10%; other syndromes include acute renal failure or thrombosis; death uncommon except with hemorrhagic syndrome |
|
Ross River virus infection, Ross River fever; epidemic polyarthritis |
Ross River virus (family Togaviridae, genus Alphavirus) |
Marsupials, including wallaby, brushtail possum, might be natural hosts; dusky rat also proposed; people, horses, ruminants, pigs, rabbits, other mammals (minor hosts) may also be a source of virus during epidemics |
Australia, South Pacific Islands |
Mosquito bites (especially Culex annulirostris and Aedes spp) |
Mild fever, arthralgia ± arthritis, headache, rash; small joints most affected but large joints can also be involved; arthralgia, myalgia, lethargy may persist for months |
|
St. Louis encephalitis |
St. Louis encephalitis virus (family Flaviviridae, genus Flavivirus) |
Wild birds, domestic fowl; rodents, bats, other mammals might also maintain viruses in South America |
Western hemisphere |
Mosquito bites (Culex tarsalis, C pipiens-quinquefasciatus complex, C nigripalpus, also reported in other genera) |
Flu-like illness sometimes followed by meningitis or encephalitis, focal neurologic signs, dysuria; more severe in elderly and those with debilitating diseases; case fatality rate of 5%–20% reported in epidemics |
|
Severe acute respiratory syndrome (SARS) |
SARS coronavirus (family Coronaviridae, genus Coronavirus) |
Bats are thought to be reservoir hosts; can also infect palm civets, raccoon dogs, cats, pigs, ferrets, rodents, nonhuman primates, other mammals |
China, southeast Asia |
Contamination of mucous membranes with respiratory droplets or virus on fomites; possibly aerosol transmission |
Fever, myalgia, headache, diarrhea, cough; viral pneumonia with rapid deterioration; case fatality rate 15% |
|
Sindbis virus disease |
Sindbis virus (family Togaviridae, genus Alphavirus) |
Birds (passeriforms suspected as main reservoirs/amplifying hosts); occasionally found in other vertebrates |
Virus widespread in Eastern hemisphere; human cases tend to occur in limited geographic regions |
Mosquito bites; Culex and Culiseta, also others |
Fever, arthritis, rash, prominent myalgia; nausea, vomiting, mild jaundice in some; joint pain can persist for months; seems to be mild or asymptomatic in most children; no fatal cases reported |
|
Tanapox |
Tanapox virus (family Poxviridae, genus Yatapoxvirus); Yaba-like disease virus may be a variant of tanapox virus |
Nonhuman primates |
Asia, Africa, and in monkey colonies |
Direct contact through broken skin; mosquitoes suspected to be vector in Africa |
Nonspecific febrile illness and papulovesicular or nodular lesions (lesions may be pruritic or tender), often on extremities; more than one or two skin lesions uncommon |
|
Tickborne encephalitis (Far eastern tickborne encephalitis, Russian spring-summer encephalitis, Central European tickborne encephalitis) |
Tickborne encephalitis virus (TBEV) (family Flaviviridae, genus Flavivirus); three subtypes: European (TBEV-Eu [least virulent]), Siberian (TBEV-Sib), Far Eastern (TBEV-FE) |
Small mammals especially rodents; also in goats, sheep, dogs, and other mammals; birds |
Eurasia; TBEV-Eu mainly Europe to former USSR; TBEV-FE mainly Asia to former USSR; TBEV-Sib mainly in Siberia |
Tick bites (mainly Ixodes ricinus and I persculatus; also other species); may be ingested in milk |
Often biphasic, with flu-like febrile illness in initial stage; neurologic signs from mild meningitis to severe encephalitis in some; myelitis or flaccid poliomyelitis-like paralysis (usually arms, shoulders, levator muscles of head); possibility of chronic and progressive forms, especially with TBEV-Sib; case fatality rate <2% (TBEV-Eu), 2%–3% (TBEV-Sib); case fatality rate 20%–30% in TBEV-FE may be based on severe cases |
|
Usutu virus infections |
Usutu virus (family Flaviviridae, genus Flavivirus) |
Birds |
Africa, Europe |
Mosquito bites (Culex spp) |
Very few cases identified: fever with rash, fever with jaundice, or meningoencephalitis |
|
Vaccinia-related poxviruses |
Vaccinia or vaccinia-like viruses (family Poxviridae, genus Orthopoxvirus) of uncertain origin |
Reservoir uncertain; found in wild rodents, cattle, horses, nonhuman primates |
Appear to be endemic in Brazil |
Direct contact |
Pox skin lesions (papules, pustules, ulcerative nodules), may be accompanied by fever, lymphadenopathy |
|
Venezuelan equine encephalomyelitis |
Venezuelan equine encephalitis virus (family Togaviridae, genus Alphavirus) |
Enzootic subtypes maintained in rodents, other small mammals, bats; epizootic subtypes amplified in equids; occasionally in other mammals and birds |
Western hemisphere; enzootic strains Florida to South America; epizootic strains emerge in South America, spread |
Mosquito bites (Aedes, Culex, and Psorophora spp); exposure to aerosolized debris from infected laboratory rodents; laboratory accidents |
Most have nonspecific febrile illness, can be followed by neurologic signs; <5% children, <1% adults progress to encephalitis with case fatality rate of 10%–35% (highest rates in children <5 yr old) |
|
Vesicular stomatitis |
Vesicular stomatitis Indiana virus, vesicular stomatitis New Jersey virus, vesicular stomatitis Alagoas virus, and Cocal virus (family Rhadboviridae, genus Vesiculovirus) |
Swine, cattle, horses; occasionally in South American camelids, sheep, and goats; also rodents; serologic evidence of infection in many wild mammals, especially bats |
North and South America; most likely not endemic north of Mexico but sporadic outbreaks |
Contact with animals or in laboratory, probably also from insect bites, including mosquitoes and biting flies (Phlebotomus spp, Lutzomyia spp, and black flies) |
Usually asymptomatic; may develop acute, febrile, flu-like illness; vesicles can be found in mouth, pharynx, or inoculation site (eg, hands); self-limiting |
|
Wesselsbron fever |
Wesselsbron virus (family Flaviviridae, genus Flavivirus) |
Ruminants, especially sheep, goats; also evidence of infection in other mammals, including lemurs; can infect birds |
Southern Africa, southeast Asia |
Mosquito bites (mainly Aedes spp, possibly others); also by contact with contaminated material |
Nonspecific febrile illness ± maculopapular rash or ocular signs in some; few cases described but seems to be self-limiting |
|
West Nile fever and neuroinvasive disease (see Equine Arboviral Encephalomyelitis) |
West Nile virus (family Flaviviridae, genus Flavivirus); lineage 1 and lineage 2 viruses are both pathogenic |
Birds are primary reservoir hosts; also affects horses, other mammals, alligators, possibly other reptiles and amphibians |
Eastern and Western hemisphere |
Mosquito bites (primarily Culex univittatus, Culex spp); also by handling infected birds or reptiles or their tissues |
Nonspecific febrile illness, occasionally with rash; some cases progress to encephalitis, meningitis, and/or acute flaccid paralysis that resembles poliomyelitis; occasionally other syndromes; worse in elderly and immunocompromised; case fatality rate ~10% in all patients with neurologic disease, but higher in elderly |
|
Western equine encephalomyelitis (see Equine Arboviral Encephalomyelitis) |
Western equine encephalomyelitis virus (family Togaviridae, genus Alphavirus) |
Birds are reservoir hosts, may also cycle in jackrabbits, rodents; equids, other mammals are incidental hosts; virus also found in reptiles, amphibians |
Americas |
Mosquito bites (Aedes, Culex, and Ochlerotatus spp); Culex tarsalis important in maintenance cycle in birds |
Nonspecific febrile illness may be followed by encephalitis in infants and children, uncommonly in adults; case fatality rate 3%–4% |
|
Yellow fever |
Yellow fever virus (family Flaviviridae, genus Flavivirus); only jungle cycle is zoonotic (people are reservoir for urban cycle) |
Nonhuman primates |
South America, Africa |
Mosquito bites (Haemagogus spp and Sabethes spp in jungle cycles in South America, Aedes spp in jungle cycles in Africa) |
Nonspecific, mild to severe febrile illness followed by liver and renal failure in some; hemorrhages (eg, epistaxis, hematemesis, melena, uterine hemorrhage) and often jaundice in severe cases; cases with hemorrhages often fatal |
|
|
|||||
|
|
|||||
|
Prion Disease |
|||||
|
Variant Creutzfeldt-Jakob disease |
Bovine spongiform encephalopathy prion |
Cattle are most important host; also infects other ruminants, cats and other felids, lemurs |
Most cases in the UK but also in many other countries |
Ingestion of bovine products, especially those contaminated with CNS tissues |
Neurodegenerative disorder similar to sporadic Creutzfeldt-Jakob disease but often in younger patients and progresses more rapidly; always fatal |
|
a Many proven zoonoses, including some relatively rare arthropodborne viral infections and helminth infections, have been omitted, as well as those diseases caused by fish and reptile toxins. |
|||||
|
b Enterotoxigenic, enteroinvasive, enteropathogenic, and enteroaggressive strains are not considered zoonotic. |
|||||